Pain is a subjective experience that cannot be directly accessed by others. Patients in clinical settings and subjects in human pain experiments are often asked to assess pain using scales, such as a numeric rating of 1–10. Although most subjects have little difficulty using these tools, some lack the necessary basic cognitive or motor skills (e.g., paralyzed patients). Also, many factors affect the way we experience pain. A 1 out of 10 for you may equal a 6 out of 10 for your brother. More objective identification of this subjective experience could be very valuable in both treatment and research. In the past couple of decades, scientists around the world have been trying to image how pain changes the brain in order to obtain a more objective read on pain and to gain insight into the mechanisms of pain processing.
Recent research into how pain changes the brain could help rheumatologists develop better treatment for pain and more effective pain-relieving drugs through objective observation of pain.
Marco L. Loggia, PhD, assistant professor of radiology at Harvard Medical School, spoke to The Rheumatologist about functional MRI (fMRI) studies exploring brain activity while patients experienced pain, either by exacerbation of their own clinical pain or by the application of external pain stimuli. fMRI is an imaging technique that can indirectly measure brain function, in addition to showing anatomic structure.
Studies Show…

“Disrupted Brain Circuitry for Pain-Related Reward/Punishment in Fibromyalgia,” the most recent study (January 2014), was published in Arthritis & Rheumatology.1 Thirty-one fibromyalgia patients and 14 controls received calibrated, cuff-pressure pain stimulus on one leg. Cuff pressure was calibrated to each subject’s pain rating of 50 on a 100-point scale. In order to investigate if and how cognitive and hedonic aspects of pain affect neural circuitry, subjects saw visual cues that pain was about to begin and end during the BOLD (blood oxygen level dependent) fMRI scan.
“It was no surprise that [fibromyalgia] patients needed less than half the pressure to have the same brain response to pain as the control subjects,” says Dr. Loggia. What was not as expected was [fibromyalgia] patients’ response to the visual cues that pain was about to start or stop.
During anticipation of both pain and pain relief, fibromyalgia patients demonstrated dampened activation in a widespread set of regions. These regions included the periaqueductal gray (i.e., a region capable of modulating pain signals from the periphery) and the ventral tegmental area (i.e., the region involved in the processing of rewards and punishment signals). Dr. Loggia says, “These dampened activations may be due to patients’ inability to perceive the experimental pain as punishing (because they suffer from their own fibromyalgia pain) and reflect their inability to engage descending pain modulation.”
Blood Flow Changes
Arterial spin labeling (ASL) can directly measure blood flow changes, and it may be superior to BOLD imaging when it comes to investigating certain aspects of clinical pain.2 “While traditional BOLD fMRI can be very effective to measure changes in brain activity due to externally applied pain,” says Dr. Loggia, “ASL seems to be more adequate to measure brain activity associated with the patients’ own clinical pain. This is because BOLD fMRI typically requests the stimulus (in this case the pain) to be rather short in duration (up to a few tens of seconds). Also, ideally the stimuli need to be repeated over and over to achieve a good signal. This, unfortunately, does not work well for clinical pain, because clinical pain cannot be turned on and off at will, and it usually lasts minutes to hours, rather than a few seconds.”
In a study using ASL, 16 patients with chronic, low back pain (cLBP) and 16 control subjects each performed identical passive, straight-leg maneuvers. The maneuvers increased the patients’ own clinical pain, but were completely painless for the controls. Increases in pain ratings by the cLBP patients were “accompanied by statistically significant increases in regional cerebral blood flow (rCBF)” in cortical areas, including the bilateral medial and dorsolateral prefrontal cortices, superior parietal lobules, S1 and S2, and the right insula; previous studies have identified these areas as being involved in processing the sensory-discriminative and affective aspects of pain.
The changes in rCBF seem to be specific with the pain maneuvers, because the pain matrix areas of the brain were activated only during the cLBP patients’ maneuvers session and not at all in the control group.
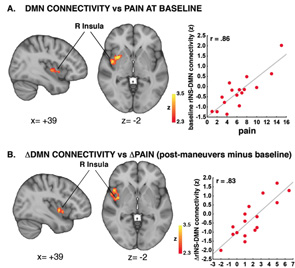
DMN
Another study looked at the connectivity of the default mode network (DMN) to build on growing evidence that chronic pain alters brain processing.3 The DMN is part of the brain that is typically more active at rest and becomes deactivated while engaging external stimuli.
The hypothesis was that changes in the connectivity between DMN and the insula (i.e., a region of the brain associated with pain processes) would occur in proportion to changes in clinical pain created by maneuvers performed on patients with cLBP. The clinical maneuvers were straight leg lifts or pelvic tilts (depending on the ability of the patient). ASL fMRI images were made before and after the maneuvers. Premaneuver values were subtracted from the postmaneuver values to determine a value for clinical pain.
Indeed, the clinical pain cLBP patients experienced did correlate to DMN-insula connectivity. “The more clinical maneuvers induced an increase in pain, the more they induced an increase in DMN-insula connectivity,” says Dr. Loggia. “And the more the insula ‘talked’ to the DMN, the more the patients reported back pain.”
After regression analysis, the researchers concluded that changes in pain were significantly associated with changes in DMN-insula connectivity. Dr. Loggia says, “The more we increased the pain, the more connectivity there was between the DMN and the insula.”
The clinical pain correlation to DMN-insula connectivity supports results that Vitaly Napadow, PhD, found in another study on fibromyalgia patients.4
“Since the same finding was observed in fibromyalgia and chronic low back pain patients, this raises the intriguing possibility that DMN-insula connectivity may be used as a marker of clinical pain across pain patient populations of different etiology,” says Dr. Loggia.
The authors cite the results as support for using ASL to evaluate clinical pain. Resting DMN connectivity may have potential as a neuroimaging biomarker to detect pain. Neuroimaging findings could also prove valuable as “surrogate endpoints” in drug research.
Summary
In sum, these studies demonstrate that neuroimaging can be a powerful tool that is capable of identifying brain changes (e.g., increases in regional cerebral blood flow or changes in the way brain regions communicate with each other) related to the experience of clinical pain. Moreover, the observation of disruption in the brain responses to pain anticipation and pain relief observed in fibromyalgia patients shows that fMRI can also provide insight into the pathological mechanisms underlying this and other pain disorders. Altogether, these neuroimaging findings turn personal, subjective experiences into objective, measurable evidence and shed new light on the brain alterations associated with chronic pain conditions.
Ann-Marie Lindstrom is an independent writer and editor based in the Tucson, Ariz., area.
What Is fMRI? See the change
According to the Center for Functional MRI at UC San Diego School of Medicine, “The discovery that MRI could be made sensitive to brain activity, as well as brain anatomy, is only about 20 years old. The essential observation was that when neural activity increased in a particular area of the brain, the MR signal also increased by a small amount. Although this effect involves a signal change of only about 1%, it is still the basis for most of the fMRI studies done today. In the simplest fMRI experiment, a subject alternates between periods of doing a particular task and a control state, such as 30 second blocks looking at a visual stimulus alternating with 30 second blocks with eyes closed. The fMRI data is analyzed to identify brain areas in which the MR signal has a matching pattern of changes, and these areas are taken to be activated by the stimulus.”
The Research
Abstract—Disrupted brain circuitry for pain-related reward/punishment in fibromyalgia1
Objective: While patients with fibromyalgia (FM) are known to exhibit hyperalgesia, the central mechanisms contributing to this altered pain processing are not fully understood. This study was undertaken to investigate potential dysregulation of the neural circuitry underlying cognitive and hedonic aspects of the subjective experience of pain, such as anticipation of pain and anticipation of pain relief.
Methods: Thirty-one FM patients and 14 controls underwent functional magnetic resonance imaging, while receiving cuff pressure pain stimuli on the leg calibrated to elicit a pain rating of ∼50 on a 100-point scale. During the scan, subjects also received visual cues informing them of the impending onset of pain (pain anticipation) and the impending offset of pain (relief anticipation).
Results: Patients exhibited less robust activation during both anticipation of pain and anticipation of relief within regions of the brain commonly thought to be involved in sensory, affective, cognitive, and pain-modulatory processes. In healthy controls, direct searches and region-of-interest analyses of the ventral tegmental area revealed a pattern of activity compatible with the encoding of punishment signals: activation during anticipation of pain and pain stimulation, but deactivation during anticipation of pain relief. In FM patients, however, activity in the ventral tegmental area during periods of pain and periods of anticipation (of both pain and relief) was dramatically reduced or abolished.
Conclusion: FM patients exhibit disrupted brain responses to reward/punishment. The ventral tegmental area is a source of reward-linked dopaminergic/γ-aminobutyric acid–releasing (GABAergic) neurotransmission in the brain, and our observations are compatible with reports of altered dopaminergic/GABAergic neurotransmission in FM. Reduced reward/punishment signaling in FM may be related to the augmented central processing of pain and reduced efficacy of opioid treatments in these patients.
References
- Loggia ML, Berna C, Kim J, et al. Disrupted brain circuitry for pain-related reward/punishment in fibromyalgia. Arthritis Rheumatol. 2014 Jan;66(1):203–212.
- Wasan AD, Loggia ML, Chen LQ, et al. Neural correlates of chronic low back pain measured by arterial spin labeling. Anesthesiology. 2011 Aug;115(2)364–374.
- Loggia ML, Kim J, Gollub RL, et al. Default mode network connectivity encodes clinical pain: An arterial spin labeling study. Pain. 2013 Jan;154(1):4–33.
- Napadow V, LaCount L, Park K, et al. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010 Aug;62(8):2545–2555.
