ACR CONVERGENCE 2021—Rheumatologists are often left in a challenging space when managing medications for patients with rheumatic diseases in relation to contraception, pregnancy and breastfeeding, especially with many novel immunosuppressants and often a dearth of pregnancy safety data.
ACR CONVERGENCE 2021—Rheumatologists are often left in a challenging space when managing medications for patients with rheumatic diseases in relation to contraception, pregnancy and breastfeeding, especially with many novel immunosuppressants and often a dearth of pregnancy safety data.
On Nov. 6 during ACR Convergence 2021, leading reproductive health experts came together to speak on this subject in a session titled Reproductive Health Update: ACR 2020 Reproductive Health Guidelines & Medication Management. Mehret Birru Talabi, MD, PhD, assistant professor of medicine in the Division of Rheumatology and Clinical Immunology, University of Pittsburgh, first presented an overview of the ACR’s Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases.1
“Many of the recommendations in the reproductive health guideline are conditional because much of the evidence is limited. Nonetheless, the [guideline] provides a comprehensive framework for reproductive healthcare and management of patients with rheumatic diseases,” she said.
Contraception Use
Dr. Birru Talabi noted contraception is safe for women with rheumatic diseases, including hormonal contraception for most patients. Patients with antiphospholipid antibody (aPL) positivity, at moderate or high titer, should avoid estrogen-containing contraception, which can further increase their thrombosis risk. However, these patients can use a copper intrauterine device (IUD), progestin IUD or progestin-only pills.
For patients with systemic lupus erythematosus (SLE) without aPL, estrogen-containing oral contraceptive pills or vaginal rings can be used. However, non-estrogen methods are recommended for patients with SLE with moderate to high disease activity. Additionally, all patients with SLE should avoid the estrogen patch.
An important point was made regarding the potential need for two contraceptive methods in patients on mycophenolate. “Because mycophenolate reduces blood levels of estrogen and progesterone and may reduce the effectiveness of oral contraception pills, the reproductive health guideline recommends patients on mycophenolate also be prescribed a highly effective contraceptive method, such as an IUD, or two other forms of contraception, such as the pill and condoms,” Dr. Birru Talabi said.
Pregnancy
Three unique populations are at higher risk for adverse outcomes in pregnancy, Dr. Birru Talabi explained. For patients with SLE, hydroxychloroquine is recommended, as well as low-dose aspirin started in the first trimester to reduce the risk of pre-eclampsia. Ro/SSA or La/SSB antibodies can be transferred across the placenta starting at around 18 weeks of gestation and may cause neonatal lupus, which ranges from transient rash to complete heart block in the neonate.
“For patients with anti-Ro/La positivity, the reproductive health guideline conditionally recommends [administering] hydroxychloroquine to reduce the incidence of heart block in the neonate and obtaining a screening fetal echocardiogram,” she said.
Lastly, patients with positive aPL or antiphospholipid antibody syndrome (APS) are also at increased risk for pregnancy complications and should be on low-dose aspirin to prevent pre-eclampsia. Anticoagulation is also recommended for obstetric patients with APS who have had multiple miscarriages or intrauterine demises, or thrombotic APS with a history of thrombosis.
“Hydroxychloroquine is conditionally recommended in patients with APS, given it has mild anticoagulant properties,” she said.
‘The reproductive health guideline recommends patients on mycophenolate also be prescribed a highly effective contraceptive method, such as an IUD, or two other forms of contraception, such as the pill & condoms.’ —Dr. Talabi
Medication Management
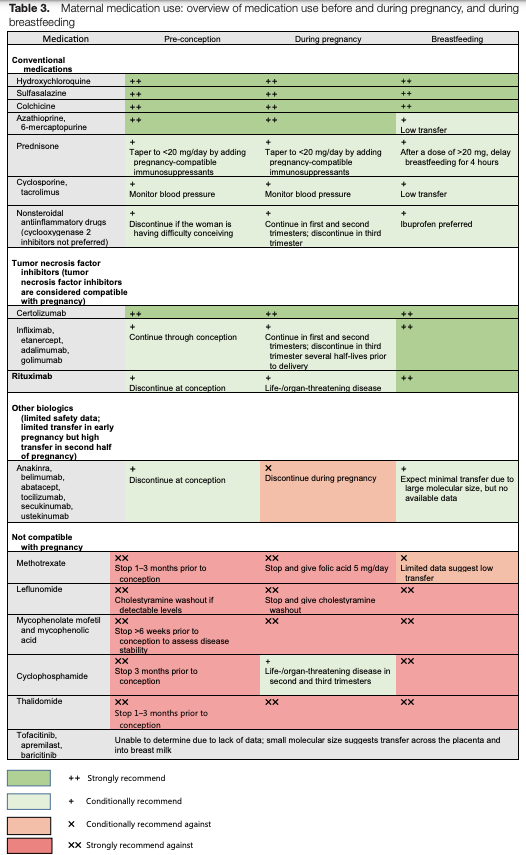
The following medications are safe to use during all phases of pregnancy and lactation: hydroxychloroquine, sulfasalazine, colchicine, azathioprine, prednisone, cyclosporine and tacrolimus. Additionally, all tumor necrosis factor (TNF) inhibitors can be used during pregnancy and lactation, although non-certolizumab TNF inhibitors are sometimes discontinued in the third trimester to avoid a potential risk of neonatal immunosuppression.
Medications contraindicated during pregnancy and breastfeeding include methotrexate, leflunomide, mycophenolate, cyclophosphamide and thalidomide. However, cyclophosphamide can be used in the second or third trimester for life- or organ-threatening disease. Small molecule agents, such as Janus kinase inhibitors (jakinibs) or apremilast, are likely to transfer across the placenta and breastmilk. These medications should not be used in pre-conception, during pregnancy or while breastfeeding (see Figure 1, below).
Figure 1: Maternal Medication Use Before & During Pregnancy, & During Breastfeeding1
One new update since the publication of the 2020 guideline was discussed: The FDA does not recommend non-steroidal anti-inflammatory drug (NSAID) use after 20 weeks of pregnancy because of the risk of oligohydramnios.
Men can safely use most medications during conception. However, cyclophosphamide and thalidomide should be discontinued before attempting to conceive. Because sulfasalazine (SSZ) can reduce sperm counts, semen analysis should be done if a man on SSZ has difficulty conceiving.
Real-World Examples
Next, Lisa Sammaritano, MD, professor of clinical medicine from the Hospital for Special Surgery, New York, and Weill Cornell Medicine, discussed applying the guideline to medication management.
Dr. Sammaritano provided an example of a patient with lupus who was breastfeeding her baby and required initiation of 25 mg of prednisone daily for post-partum flare. For doses of prednisone greater than 20 mg per day, patients should delay breastfeeding—or pump and discard—for four hours after taking the dose. She emphasized considering the addition of steroid-sparing, breastfeeding-compatible medications if prednisone cannot be quickly tapered.
Non-TNF biologics (e.g., anakinra, belimumab, abatacept, tocilizumab, secukinumab, ustekinumab) should be discontinued during pregnancy due to limited safety data. However, due to their large molecular size, these medications result in minimal transfer to breastmilk and are compatible with breastfeeding.
“Because IgG placental transfer only begins at 16 weeks of gestation, TNF inhibitors can be used safely through the end of the second trimester,” Dr. Sammaritano explained. “These are often discontinued at 30 weeks, when transfer significantly increases—except certolizumab.”
If needed, these medications can be continued throughout the pregnancy, with consideration of the risk of neonatal immunosuppression.
In another example, she emphasized that the most effective form of contraception for all rheumatic disease patients is long-acting reversible contraception, such as an IUD or progestin implant. Estrogen-containing contraception must be avoided in patients with aPL positivity and/or very active lupus. Additionally, depot-medroxyprogesterone acetate (DPMA or depo) should be avoided in patients who are aPL positive given limited data suggesting an increased risk of thrombosis. Because DMPA also reduces bone mineral density, its use may not be ideal in patients who have risk factors for osteoporosis.
Question & Answer
During the question-and-answer session, clinical dilemmas were discussed that often have absence of rigorous evidence to guide practice. For instance, the precise dosing of hydroxychloroquine in pregnant patients who are SSA/SSB positive and necessary frequency of fetal echocardiogram monitoring in these patients is unknown.
 Mithu Maheswaranathan, MD, completed his fellowship in rheumatology at Duke University in Durham, N.C., where he is currently a clinical instructor in the Division of Rheumatology and Immunology. He can be contacted on Twitter (@MithuRheum).
Mithu Maheswaranathan, MD, completed his fellowship in rheumatology at Duke University in Durham, N.C., where he is currently a clinical instructor in the Division of Rheumatology and Immunology. He can be contacted on Twitter (@MithuRheum).
Reference
- Sammaritano LR, Bermas BL, Chakravarty EE, et al. 2020 American College of Rheumatology Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases. Arthritis Rheumatol. 2020 Apr;72(4):529–556. Epub 2020 Feb 23.