CARRA was established at a critical time for pediatric rheumatology, which was clearly endangered as a specialty: In the late 1990s, fewer than six fellows graduated from pediatric rheumatology fellowships each year in the entire U.S., and almost half of U.S. medical schools did not have a pediatric rheumatologist on their faculty.1
To foster academic career development, increase the workforce and facilitate widespread collaborative participation in research, all pediatric rheumatologists, regardless of their scientific or academic accomplishments, were invited to participate in the inaugural CARRA Annual Meeting in 2002. Engagement of early career investigators was emphasized as another crucial component for the future of academic pediatric rheumatology.
The Arthritis Foundation provided initial small infrastructure grants to CARRA that helped launch CARRA activities. Over the years, these grants have grown considerably, enabling the nascent network to extend its scope of activities and thrive. Support for the annual meeting, including participant travel costs, was made possible through meeting grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the American College of Rheumatology (ACR) and The Wasie Foundation, as well as corporate sponsors. Most of the funding for specific research studies and clinical trials came from NIAMS, the Arthritis Foundation, Lupus Foundation of America (LFA), Cure Juvenile Myositis (CureJM), the ACR and Friends of CARRA, with the Duke Clinical Research Institute (DCRI) functioning as the data coordinating center for the CARRA Registry and other clinical trials.
The Growth Years: 2006–2012
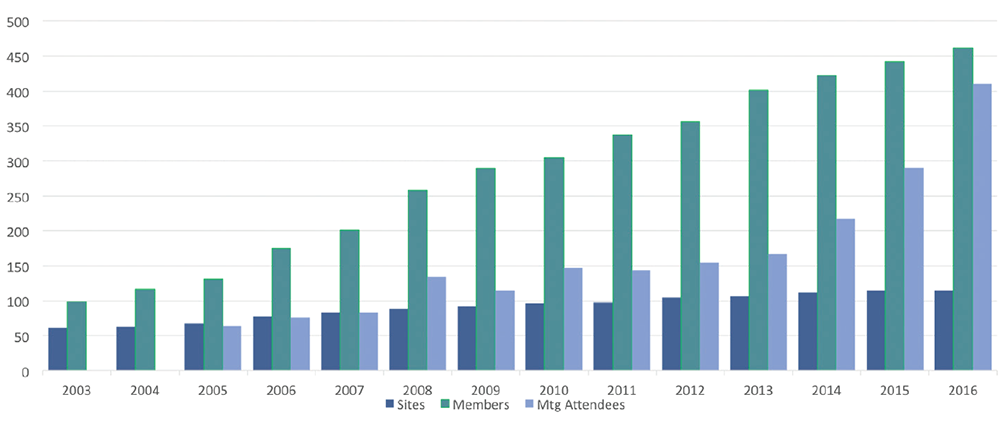
Over the years, CARRA has grown to include more than 110 sites in the U.S. and Canada, and greater than 400 members (see Figure 1). In excess of 60 funded projects totaling over $60 million, including three major NIAMS-funded multicenter clinical trials, have been awarded to CARRA investigators since 2006 (see Figure 2).
(click for larger image)
Figure 1: CARRA Membership and Meeting Attendance
CARRA’s growth over its lifespan as shown by the number of sites (dark blue), members (teal) and meeting attendees (light blue).
(click for larger image)
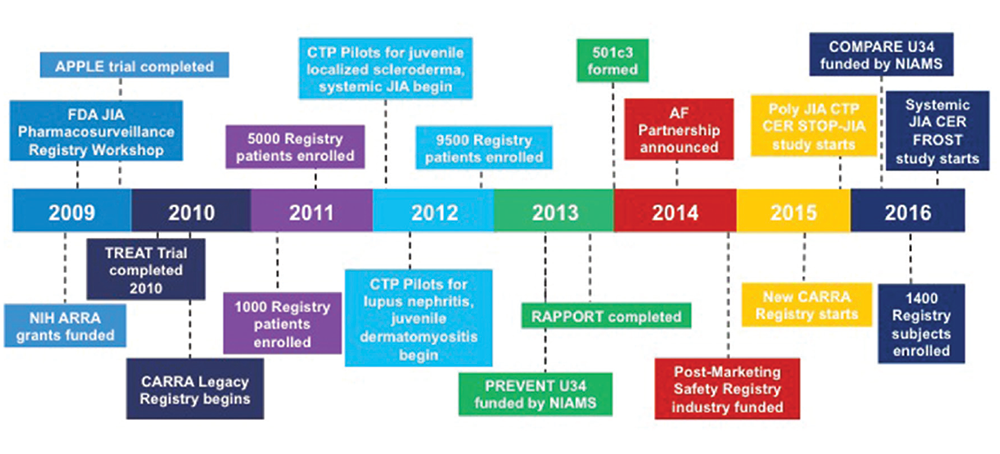
Figure 2: CARRA Timeline (2009–present)
Significant recent events for the CARRA organization since 2009.
The three NIH-funded clinical trials that have been successfully completed are: 1) the first multicenter randomized controlled trial in pediatric lupus—the Atherosclerosis Prevention in Pediatric Lupus Erythematosus (N01-AR-2-2265, APPLE) trial, which tested the efficacy of atorvastatin in preventing premature carotid intimal medial thickening in children with SLE;2 2) the Trial of Early Aggressive Therapy in polyarticular JIA (R01-AR049762, TREAT-JIA), a randomized controlled trial comparing treatment with methotrexate vs. methotrexate/steroids/etanercept;3 and 3) the Randomized Placebo Phase Trial of Rilonacept in systemic JIA (N01 AR 700015, RAPPORT).4

