These trials, in addition to many other clinical, translational and observational studies, created momentum for the network, operationalized a burgeoning culture of research and established CARRA as an important research organization. Membership expanded to include non‑pediatric rheumatologists who were engaged in research in pediatric rheumatic diseases, including laboratory scientists, psychologists, adult rheumatologists and epidemiologists. In addition, a research coordinator network was created.
Two NIH awards funded through the American Recovery and Reinvestment Act (ARRA) in 2009 were a critical turning point for CARRA.
(click for larger image)
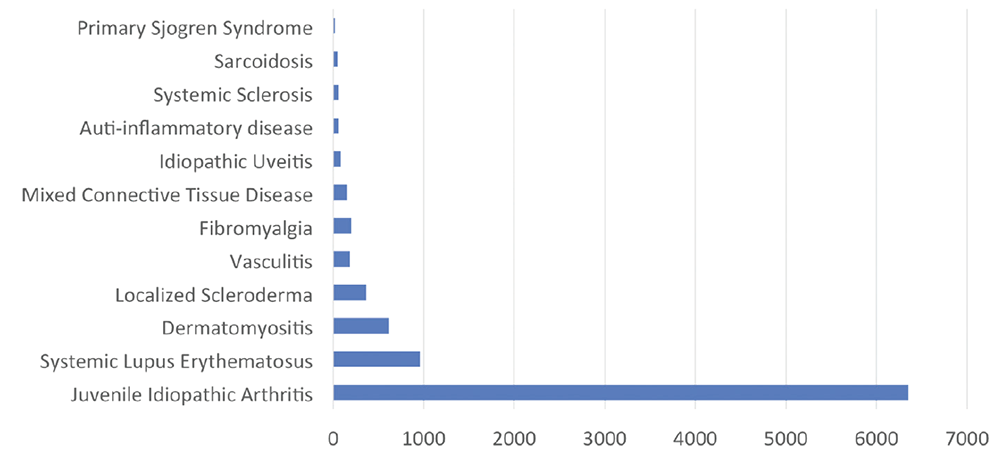
Figure 3: CARRA Legacy Registry Snapshot of Enrollment by Disease
Enrollment numbers are shown by disease in the CARRA Legacy Registry.
One award, a Grand Opportunities Grant (RC2-AR-058934; CARRA: Accelerating Toward an Evidence Based Culture in Pediatric Rheumatology), allowed the development of what is now referred to as the CARRA Legacy Registry, including the enrollment of more than 9,500 patients with pediatric rheumatic diseases (see Figure 3), the engagement of 60 CARRA sites and more than 250 investigators, as well as their coordinators, and the creation of the registry’s 21 CFR Part 11 FDA-compliant, federated open-source informatics platform using the InForm electronic data capture system warehoused in i2b2 (Informatics Integrating Bench to Bedside).
CARRA was established at a critical time for pediatric rheumatology, which was clearly endangered as a specialty: In the late 1990s, fewer than six fellows graduated from pediatric rheumatology fellowships each year in the entire U.S.
The CARRA Registry finally made real the possibility of offering participation in research to every child affected by rheumatic diseases as was envisioned by the founders. The other NIH grant, a Challenge Grant (1RC1AR058605-01, Comparative Effectiveness Research in Pediatric Rheumatic Diseases: Leveraging CARRA), funded the development of standardized consensus-derived treatment plans (Consensus Treatment Plans [CTPs]) in four pediatric rheumatic diseases: systemic juvenile idiopathic arthritis (JIA), pediatric lupus nephritis, juvenile dermatomyositis and localized scleroderma.5-9
The CARRA CTPs are designed to facilitate comparative effectiveness research by allowing researchers to study consensus-derived, standardized treatments for pediatric rheumatic diseases used in a real-world observational setting and using the CARRA Registry for data collection. Pilot studies testing the feasibility of this approach were funded by the Arthritis Foundation, LFA and CureJM Foundation and completed successfully.10-13
The Legacy Registry continues to be a rich resource of information, resulting in multiple abstracts and publications, despite closing in 2014.
The New CARRA Registry & Current Initiatives
A new iteration of the Registry was launched in July 2015, creating a disease-specific pharmacosurveillance registry that meets industry Phase IV requirements, operationalizing a concept introduced at an FDA workshop in 2009 to improve the safety surveillance of new products brought to market to treat JIA.14,15 The Registry will systematically follow at least 10,000 children and adolescents with JIA for 10 or more years regardless of medication exposure.

