Pediatric rheumatology was formally recognized as a specialty in 1991 by the American Board of Pediatrics.
Yukiko Kimura, MD
Pediatric rheumatology was formally recognized as a specialty in 1991 by the American Board of Pediatrics. Prior to this time, children with rheumatic diseases were treated by a hodgepodge of providers. In addition to providers who had training as pediatric rheumatologists, general pediatricians, adult rheumatologists, allergist-immunologists, orthopedists, pediatric infectious disease specialists and others treated children with rheumatic diseases. As a result, there was little advocacy or research in the field and little consistency in treatment approaches.
It was not until the late 20th century, with clear successes treating children with cancer through the development of several pediatric oncology networks working to standardize, protocolize and study treatments for all children with specific cancers, that the importance of evidence-based treatment approaches was recognized by healthcare providers and families.
The passage of national legislation mandating the study of new pharmaceuticals in children as part of FDA approval packages and the success of methotrexate and later anti-TNF agents in the treatment of rheumatoid arthritis also highlighted the need and opportunities for ongoing clinical and translational research in the field of pediatric rheumatology.
CARRA: The Beginning (2000–2007)
The Childhood Arthritis & Rheumatology Research Alliance (CARRA) began in 2000 as a grassroots initiative among a small number of pediatric rheumatologists in the U.S. and Canada seeking to establish an inclusive, collaborative research network. The overall mission and supporting guiding principles that outlined scientific, leadership and operational elements were crafted at a meeting in 2001, and the initial scientific agenda and criteria for studies to be undertaken by the network were established in 2002.
CARRA was envisioned as a network that would enable widespread research participation, thereby ensuring the future of academic pediatric rheumatology, which was endangered at the time. Three crucial components were identified at the meeting that continue to form the bedrock of the current organization:
- An organizational structure that includes rotating leadership positions and a steering committee elected by members to specific terms with term limits;
- Disease-based research committees led by rotating elected committee chairs forming the heart of the organization and encompassing the breadth of diseases and interests within the pediatric rheumatology community; and
- Commitment to include all pediatric rheumatology investigators and sites to further research accessibility and make it possible for all children diagnosed with rheumatic diseases and their families to participate in research studies.
The annual face-to-face scientific working meeting then became a crucial component of CARRA’s operation, fostering a culture of collaboration and research among the entire pediatric rheumatology community.
CARRA was established at a critical time for pediatric rheumatology, which was clearly endangered as a specialty: In the late 1990s, fewer than six fellows graduated from pediatric rheumatology fellowships each year in the entire U.S., and almost half of U.S. medical schools did not have a pediatric rheumatologist on their faculty.1
To foster academic career development, increase the workforce and facilitate widespread collaborative participation in research, all pediatric rheumatologists, regardless of their scientific or academic accomplishments, were invited to participate in the inaugural CARRA Annual Meeting in 2002. Engagement of early career investigators was emphasized as another crucial component for the future of academic pediatric rheumatology.
The Arthritis Foundation provided initial small infrastructure grants to CARRA that helped launch CARRA activities. Over the years, these grants have grown considerably, enabling the nascent network to extend its scope of activities and thrive. Support for the annual meeting, including participant travel costs, was made possible through meeting grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the American College of Rheumatology (ACR) and The Wasie Foundation, as well as corporate sponsors. Most of the funding for specific research studies and clinical trials came from NIAMS, the Arthritis Foundation, Lupus Foundation of America (LFA), Cure Juvenile Myositis (CureJM), the ACR and Friends of CARRA, with the Duke Clinical Research Institute (DCRI) functioning as the data coordinating center for the CARRA Registry and other clinical trials.
The Growth Years: 2006–2012
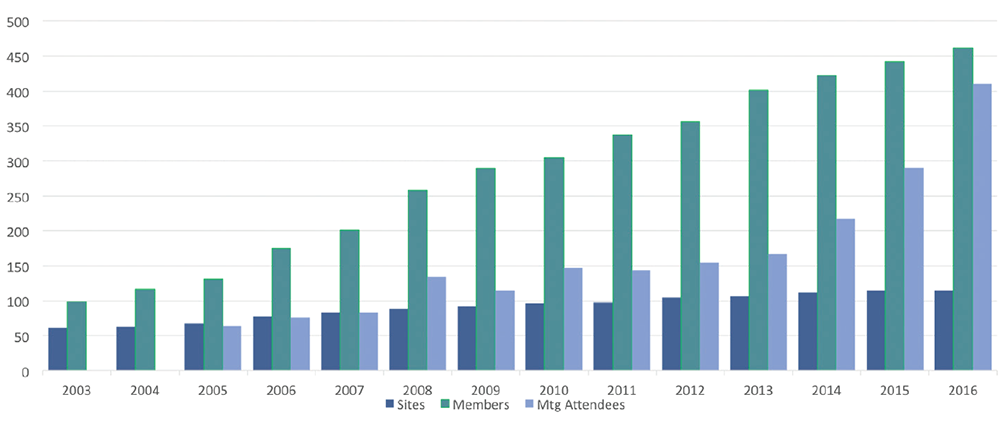
Over the years, CARRA has grown to include more than 110 sites in the U.S. and Canada, and greater than 400 members (see Figure 1). In excess of 60 funded projects totaling over $60 million, including three major NIAMS-funded multicenter clinical trials, have been awarded to CARRA investigators since 2006 (see Figure 2).
(click for larger image)
Figure 1: CARRA Membership and Meeting Attendance
CARRA’s growth over its lifespan as shown by the number of sites (dark blue), members (teal) and meeting attendees (light blue).
(click for larger image)
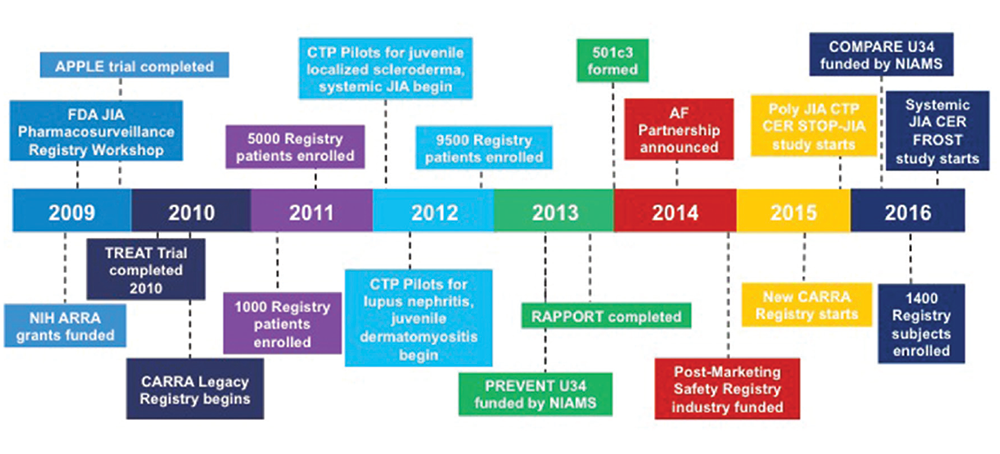
Figure 2: CARRA Timeline (2009–present)
Significant recent events for the CARRA organization since 2009.
The three NIH-funded clinical trials that have been successfully completed are: 1) the first multicenter randomized controlled trial in pediatric lupus—the Atherosclerosis Prevention in Pediatric Lupus Erythematosus (N01-AR-2-2265, APPLE) trial, which tested the efficacy of atorvastatin in preventing premature carotid intimal medial thickening in children with SLE;2 2) the Trial of Early Aggressive Therapy in polyarticular JIA (R01-AR049762, TREAT-JIA), a randomized controlled trial comparing treatment with methotrexate vs. methotrexate/steroids/etanercept;3 and 3) the Randomized Placebo Phase Trial of Rilonacept in systemic JIA (N01 AR 700015, RAPPORT).4
These trials, in addition to many other clinical, translational and observational studies, created momentum for the network, operationalized a burgeoning culture of research and established CARRA as an important research organization. Membership expanded to include non‑pediatric rheumatologists who were engaged in research in pediatric rheumatic diseases, including laboratory scientists, psychologists, adult rheumatologists and epidemiologists. In addition, a research coordinator network was created.
Two NIH awards funded through the American Recovery and Reinvestment Act (ARRA) in 2009 were a critical turning point for CARRA.
(click for larger image)
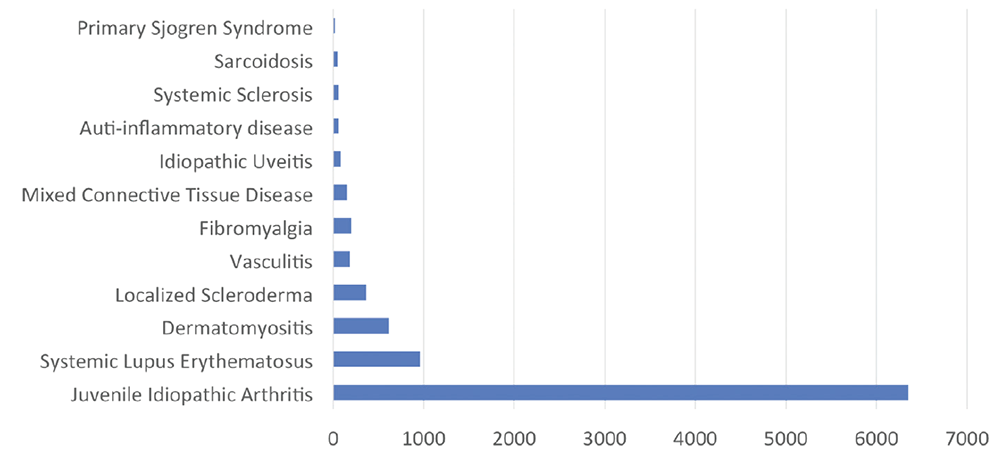
Figure 3: CARRA Legacy Registry Snapshot of Enrollment by Disease
Enrollment numbers are shown by disease in the CARRA Legacy Registry.
One award, a Grand Opportunities Grant (RC2-AR-058934; CARRA: Accelerating Toward an Evidence Based Culture in Pediatric Rheumatology), allowed the development of what is now referred to as the CARRA Legacy Registry, including the enrollment of more than 9,500 patients with pediatric rheumatic diseases (see Figure 3), the engagement of 60 CARRA sites and more than 250 investigators, as well as their coordinators, and the creation of the registry’s 21 CFR Part 11 FDA-compliant, federated open-source informatics platform using the InForm electronic data capture system warehoused in i2b2 (Informatics Integrating Bench to Bedside).
CARRA was established at a critical time for pediatric rheumatology, which was clearly endangered as a specialty: In the late 1990s, fewer than six fellows graduated from pediatric rheumatology fellowships each year in the entire U.S.
The CARRA Registry finally made real the possibility of offering participation in research to every child affected by rheumatic diseases as was envisioned by the founders. The other NIH grant, a Challenge Grant (1RC1AR058605-01, Comparative Effectiveness Research in Pediatric Rheumatic Diseases: Leveraging CARRA), funded the development of standardized consensus-derived treatment plans (Consensus Treatment Plans [CTPs]) in four pediatric rheumatic diseases: systemic juvenile idiopathic arthritis (JIA), pediatric lupus nephritis, juvenile dermatomyositis and localized scleroderma.5-9
The CARRA CTPs are designed to facilitate comparative effectiveness research by allowing researchers to study consensus-derived, standardized treatments for pediatric rheumatic diseases used in a real-world observational setting and using the CARRA Registry for data collection. Pilot studies testing the feasibility of this approach were funded by the Arthritis Foundation, LFA and CureJM Foundation and completed successfully.10-13
The Legacy Registry continues to be a rich resource of information, resulting in multiple abstracts and publications, despite closing in 2014.
The New CARRA Registry & Current Initiatives
A new iteration of the Registry was launched in July 2015, creating a disease-specific pharmacosurveillance registry that meets industry Phase IV requirements, operationalizing a concept introduced at an FDA workshop in 2009 to improve the safety surveillance of new products brought to market to treat JIA.14,15 The Registry will systematically follow at least 10,000 children and adolescents with JIA for 10 or more years regardless of medication exposure.
Unlike historical drug-specific safety registries, which are limited in scope and participation, this format allows a more robust estimation of the risk of rare long-term adverse events, such as malignancies and opportunistic infections in exposed and non-exposed individuals. It also allows improved understanding of long-term outcomes in real-life settings with mechanisms in place to continue follow-up into adulthood even as patients transition out of the pediatric age range.
The FDA endorsed this approach to pharmacosurveillance, and several companies have engaged CARRA to use the Registry to satisfy post-marketing requirements.15 As of summer 2016, the CARRA Registry had enrolled almost 1,500 patients, mostly with polyarticular and systemic JIA. Other pediatric rheumatic diseases will be added as funding allows, beginning with SLE in 2016.
The “new” CARRA Registry has become the preferred data-collection vehicle for all large multicenter CARRA studies, which are not limited to pharmacosurveillance, observational and natural history studies (see Figure 4). Ongoing studies of comparative effectiveness in an observational setting are feasible and economical compared with randomized active comparator trials and provide a research model for studies in other rare diseases. One such example, STOP-JIA (Start Time Optimization of biologics in Poly-JIA), funded by the Patient-Centered Outcomes Research Institute (PCORI), will compare the effectiveness of the polyarticular JIA CTPs to answer the pressing clinical question of when biologics should be started in a patient with new-onset polyarticular JIA.
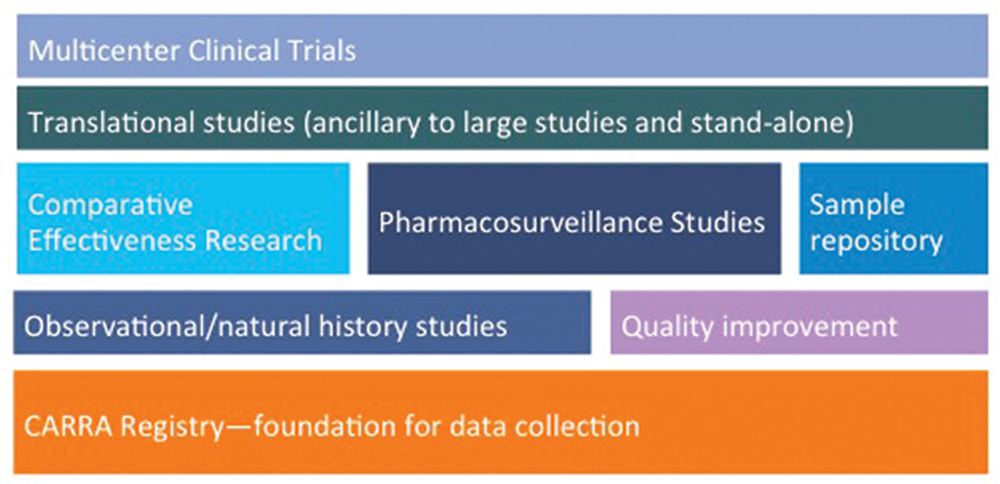
(click for larger image)
Figure 4: Planning & Building the CARRA Research Portfolio on the Foundation of the CARRA Registry
The breadth of the current and planned research initiatives using the CARRA Registry as the foundation for data collection.
Similarly designed is FROST (First-Line Options for Systemic JIA Treatment), which is due to begin fall 2016 and will compare the CARRA systemic JIA CTPs in children with new-onset systemic JIA.
Because these are observational studies with standard of care treatments, data collection as outlined in the general CARRA Registry protocol and utilize existing site contracts, operationalizing these studies is relatively easy and efficient compared with starting stand-alone studies.
Companion translational studies leveraging the biospecimen infrastructure within the network are being implemented for both STOP-JIA and FROST, focusing on patients before and after starting biologic or DMARD therapy, and aim to identify biomarkers that predict response and non-response, as well as aiding in diagnosis.
Moving forward, translational studies and appropriate biospecimen collection will be a routine part of CARRA studies, creating a robust resource of highly phenotyped samples linked to longitudinal clinical data to fuel other translational and basic investigation. Investigator-initiated and industry-sponsored randomized clinical trials are also in development and will take advantage of the CARRA Registry infrastructure and network.
Recent initiatives to increase patient and stakeholder engagement have broadened perspectives of CARRA investigators, including molding and changing critical aspects of studies and research workgroups. Parents and patients make up an increasing presence at the CARRA annual meeting, vocally participating in workgroup activities and serving on study teams. Patient study team members for STOP-JIA are helping guide study design and operations, developing patient facing materials, and leading a stakeholder advisory panel.
In addition, CARRA is part of the PARTNERS (Patients, Advocates and Rheumatology Teams Network for Research and Service) Consortium funded by PCORI as a patient-powered research network that is part of PCORnet, the National Patient-Centered Clinical Research Network. One of CARRA’s goals for the future is to embed stakeholder engagement into all CARRA research activities.
CARRA became a 501c3 tax exempt nonprofit organization in late 2014. A Board of Directors was created along with administrative infrastructure for CARRA Inc., which includes an executive director, research operations director and other personnel to work with Steering Committee leadership to operationalize and move CARRA’s mission forward. Becoming a legal organization allows CARRA to negotiate and execute contracts directly with pharmaceutical companies, vendors and the data coordinating center (DCRI).
Another critical factor in CARRA’s recent growth has been the 2015 expansion of the longstanding relationship with the Arthritis Foundation, which has provided increased funding and in-kind operational support, allowing for long-term strategic planning and development of new programs. Examples of the new programs include a small grant program to help fund collaborative early stage projects emerging from the workgroups, modest salary support for steering committee members, a pediatric resident scholarship program at the annual meeting, a CARRA Registry internship program for early career investigators, the addition of data collection for several diseases to the registry (SLE, JDM, localized scleroderma) and a CARRA early investigator program, to name a few examples.
Future Evolution & Challenges
As CARRA thrives as a research organization and continues to grow larger and more complex, major challenges include maintaining its organizational transparency and democratic governance structure, improving member and stakeholder engagement and satisfaction, finding innovative methods of communicating with all members and the public, and ensuring leadership succession.
As a network of academic clinicians and researchers from many institutions and organizations with different levels of existing research support, interests and skills, as well as widely divergent levels of research experience, inherent challenges exist in improving research efficiency and increasing the capacity and diversity of the CARRA research portfolio. CARRA, working with DCRI, is invested in streamlining research through the use of Master Contracts, reliant IRB mechanisms (IRBrely), standardized e-consenting, more extensive use of e-PROs, a standardized data dictionary across studies and electronically facilitated data sharing.
Increasing collaborations with the international pediatric rheumatology community is critical to making major scientific advances in rare diseases. CARRA is committed to facilitating international research efforts, but this increases the complexity of the research process. Working collaboratively with current funders and continuing to develop new funding streams is also important to ensure the organization’s financial sustainability.
The challenge to the pediatric rheumatology community is maintaining the principles that have allowed CARRA to grow & thrive while translating its successes into important scientific advances & discoveries for patients & families going forward.
Lastly, the current leadership of CARRA were all members of the original cohort of pediatric rheumatologists that founded CARRA and will need to be replaced. Given the low numbers of pediatric rheumatologists trained in the late 1990s and the significant increase in the numbers of pediatric rheumatology fellows now being trained, the pediatric rheumatology community will need to rely on younger members with less experience to assume leadership positions within the organization. It is, therefore, a major imperative for current CARRA leaders to nurture and foster future generations.
With that in mind, the Early Investigator Committee, whose leadership serves on the Steering Committee, was developed. Responsibilities of the Early Investigator Committee include encouraging promising young investigators to become involved in all levels of CARRA activities and fostering engagement with international counterparts. In addition, there are formal leadership development programs for all Steering Committee members, as well as informal mentoring of each committee chair by a member of the CARRA executive team.
In summary, the future of academic pediatric rheumatology seems brighter than ever, with a growing workforce and a variety of effective medications to treat pediatric rheumatic diseases and prevent life-altering disability; however, there is still much to be learned.
The culture of transparency and scientific collaboration that has been fostered and maintained by CARRA has led to its current level of success and promises to change clinical practice and improve health outcomes. The challenge to the pediatric rheumatology community is maintaining the principles that have allowed CARRA to grow and thrive while translating its successes into important scientific advances and discoveries for patients and families going forward.
Yukiko Kimura, MD, is chair of the Childhood Arthritis & Rheumatology Research Alliance (CARRA) and chief of pediatric rheumatology at Joseph M. Sanzari Children’s Hospital, Hackensack University Medical Center, Hackensack, N.J.
Laura E. Schanberg, MD, is immediate past chair of CARRA and co-chief of pediatric rheumatology at Duke University Medical Center, Durham, N.C.
References
- Cassidy JT, Athreya B. Pediatric rheumatology: Status of the subspecialty in United States medical schools. Arthritis Rheum. 1997 Jun;40(6):1182.
- Schanberg LE, Sandborg C, Barnhart HX, et al. Premature atherosclerosis in pediatric systemic lupus erythematosus: Risk factors for increased carotid intima-media thickness in the atherosclerosis prevention in pediatric lupus erythematosus cohort. Arthritis Rheum. 2009 May;60(5):1496–1507.
- Wallace CA, Giannini EH, Spalding SJ, et al. Trial of early aggressive therapy in polyarticular juvenile idiopathic arthritis. Arthritis Rheum. 2012 Jun;64(6):2012–2021.
- Ilowite NT, Prather K, Lokhnygina Y, et al. Randomized, double-blind, placebo-controlled trial of the efficacy and safety of rilonacept in the treatment of systemic juvenile idiopathic arthritis. Arthritis Rheumatol. 2014 Sep;66(9):2570–2579.
- DeWitt EM, Kimura Y, Beukelman T, et al. Consensus treatment plans for new-onset systemic juvenile idiopathic arthritis. Arthritis Care Res (Hoboken). 2012 Jul;64(7):1001–1010.
- Li SC, Torok KS, Pope E, et al. Development of consensus treatment plans for juvenile localized scleroderma: A roadmap toward comparative effectiveness studies in juvenile localized scleroderma. Arthritis Care Res (Hoboken). 2012 Aug;64(8):1175–1185.
- Huber AM, Giannini EH, Bowyer SL, et al. Protocols for the initial treatment of moderately severe juvenile dermatomyositis: Results of a Children’s Arthritis and Rheumatology Research Alliance Consensus Conference. Arthritis Care Res (Hoboken). 2010 Feb;62(2):219–225.
- Huber AM, Robinson AB, Reed AM, et al. Consensus treatments for moderate juvenile dermatomyositis: Beyond the first two months. Results of the second Childhood Arthritis and Rheumatology Research Alliance consensus conference. Arthritis Care Res (Hoboken). 2012 Apr;64(4):546–553.
- Mina R, von Scheven E, Ardoin SP, et al. Consensus treatment plans for induction therapy of newly diagnosed proliferative lupus nephritis in juvenile systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2012 Mar;64(3):375–383.
- Kimura Y, Beukelman T, Morgan E, et al. Results from the Childhood Arthritis and Rheumatology Research Alliance Systemic JIA Consensus Treatment Plans Pilot Study. Arthritis Rheum. 2015 Oct;67(S10):959.
- Reed A, Huber A, Tomlinson G, et al. CARRA dermatomyositis CPT pilot study. Pediatr Rheumatol. 2016 Jul;14(Suppl 1):P23.
- Cooper JC, Eberhard BA, Punaro M, A, et al. A pilot study of consensus treatment plans for induction therapy in childhood proliferative lupus nephritis. Arthritis Rheum. 2016; Submitted.
- Li SC, Torok KS, Hong SD, et al. Initial results of a pilot juvenile localized scleroderma comparative effectiveness study [abstract]. Arthritis Rheumatol. 2015 Oct;67(Suppl 10):3095.
- Smith MY, Sobel RE, Wallace CA. Monitoring the long-term safety of therapies for children with juvenile idiopathic arthritis: Time for a consolidated patient registry. Arthritis Care Res (Hoboken). 2010 Jun;62(6):800–804.
- Lionetti G, Kimura Y, Schanberg LE, et al. Using registries to identify adverse events in rheumatic diseases. Pediatrics. 2013;132(5):e1384–e1394.

