For example, it is possible that a very large number of additional variants, with increasingly smaller association strength, are contributing to disease susceptibility, in which case even larger studies may be required to identify these loci. Another possibility is that much of the missing heritability reflects the action of a large number of rare variants that have not been specifically targeted in recent GWAS and other genetic studies. These variants will require different approaches for identification, such as high-throughput DNA sequencing. Another category of genetic variation that has not been specifically targeted or captured in most recent genetic studies are structural variants, such as copy-number variation, as has been previously demonstrated for C4 and FcgR genes in SLE.
Epigenetics and the Environment
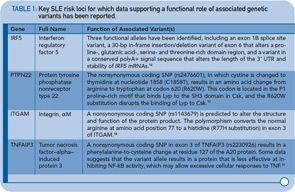
Recent data also provide compelling support for a role of epigenetic factors in SLE. Epigenetics refers to inherited changes in gene expression caused by mechanisms other than DNA base sequence changes. The most well understood type of epigenetic factor is DNA methylation, which has been shown to play a role in a variety of human processes, such as X chromosome inactivation and certain cancers. Recent work by Javierre et al suggests that differences in the DNA methylation status of genes may explain, at least in part, the discordance observed in some identical twins that are discordant for SLE.14 More specifically, they found that twins with SLE were characterized by lower levels of DNA methylation overall, including approximately 50 genes with established roles in immune responses, cytokine production, and cell activation. Previous research has also implicated the importance of DNA methylation in SLE. Given the strong impact of environmental factors on the DNA methylation status of genes, as strikingly illustrated by the Agouti mouse model in which changes in the methylation content of the diet have profound effects on the coat color and other phenotypic characteristics (e.g., obesity, diabetes), epigenetic mechanisms could potentially provide a missing link between genetic and environmental risk factors for SLE. As with many of the aforementioned unanswered questions, epigenetics will likely be an intense focus of future investigation.
The extent to which recently identified SLE susceptibility loci appear to be relevant to other autoimmune disorders, and vice versa, has reinforced the importance of family history in the evaluation of SLE patients.

Although much work remains to be done to fully define the genes and epigenetic factors that contribute to disease risk and outcome in SLE, it is interesting to consider how this information may contribute to clinical practice in the future. The extent to which recent genetics studies have informed our understanding of disease mechanisms in SLE is impressive, and our knowledge will continue to develop as we more fully elucidate the genetic underpinnings of this and related disorders. Such information has great potential to accelerate the identification of targets for novel therapies, as reviewed recently by Plenge and Raychaudhuri.15 One can also envision the incorporation of genetic information into a new generation of diagnostic or classification criteria, which would be informed by knowledge about specific disease subsets that are more homogeneous in terms of disease etiology, prognosis, and treatment response. Certainly, the extent to which recently identified SLE susceptibility loci appear to be relevant to other autoimmune disorders, and vice versa, has reinforced the importance of family history in the evaluation of SLE patients. It is clear that a family history of a wide range of autoimmune diseases may have relevance for an individual patient being evaluated for a possible diagnosis of SLE.

