It’s possible that a differential susceptibility exists among lymphocyte subpopulations to CS, explaining the high rate of relapse or the presence of subclinical vascular inflammation in some individuals. This theory is the subject of ongoing investigation.
The IL-6 pathway, overexpressed in GCA, represents a potential target for therapy. IL-6 has diverse biological functions depending on its target cell. During physiologic inflammatory responses, IL-6 triggers the synthesis of acute-phase proteins, promotes the transition from acute to chronic inflammation, and facilitates the development of specific immunity. IL-6 modulates the activation, proliferation and differentiation of T cells (including Th17 and regulatory T cells), and induces cells of the monocyte, endothelial and stromal lineages to acquire a “proinflammatory” phenotype.
In GCA patients, the IL-6 gene is actively transcribed within inflamed arteries, and the IL-6 concentration is elevated in peripheral circulation. Serum IL-6 levels parallel disease activity and decline with adequate CS treatment.15 Therefore, IL-6 signaling inhibition may ameliorate large-vessel vasculitis in GCA through different mechanisms that include altering upstream differentiation of autoreactive lymphocytes, promoting the generation of regulatory T cells, and/or deamplifying downstream aspects of the innate inflammatory network.
In published reports, approximately two dozen of patients with GCA have received the IL-6 receptor (IL-6R) antagonist tocilizumab (TCZ) with encouraging preliminary results.16 To rigorously evaluate the safety and efficacy of IL-6R blockade in GCA, a Phase III, multicenter, randomized, double-blind, placebo-controlled study is currently ongoing.17
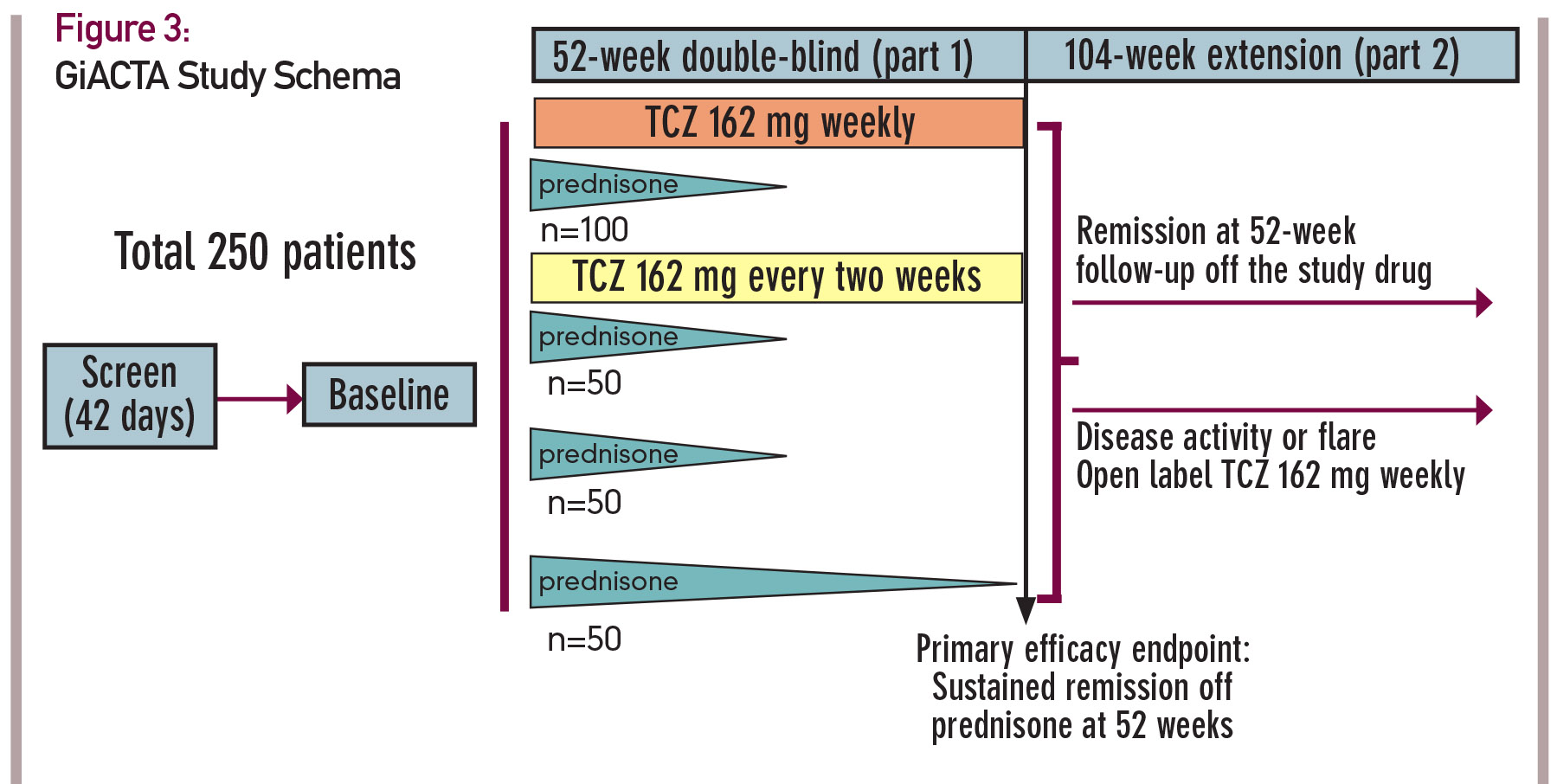
In this trial, 250 patients will be enrolled and assigned to one of four treatment arms. The design of this trial, known as GiACTA, is shown in Figure 3 (see p. 23). GiACTA consists of a 52-week blinded period, followed by 104 weeks of safety extension. Two subcutaneous doses of TCZ are being compared with placebo. All patients also receive background CS therapy. Three groups follow a prespecified prednisone taper regimen over 26 weeks, and a fourth group receives a 52-week prednisone taper as monotherapy. The primary endpoint of the clinical trial is glucocorticoid-free remission at 52 weeks after randomization.
Of note, this research endeavor will also serve as the platform for important ancillary substudies that include imaging investigations (e.g., PET/CT and vascular ultrasound), mechanistic analysis and, for the first time in this disease, genome-wide gene expression profiling.
At the time of this writing, the GiACTA trial is more than 80% enrolled; 210 patients with GCA are already participating in this trial. The trial is scheduled to complete enrollment in late spring 2015.