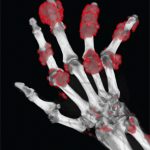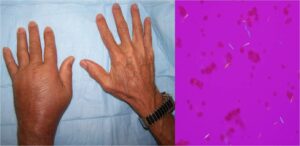
Diffuse swelling of the dorsum of the left hand is evident in this patient with acute gouty arthritis (left panel). A left wrist aspirate contained extracellular needle-shaped crystals that exhibited strong negative birefringence with compensated polarized light microscopy (right panel). This diffuse hand swelling arises from both wrist arthritis and dorsal extensor tenosynovitis.
PHILADELPHIA—Approximately 60 research abstracts on gout were accepted for presentation at ACR Convergence 2022, including two plenary abstract presentations. It is exciting to see a wealth of research on gout being undertaken worldwide. Here, we highlight important points from seven of these studies.
1. Abstract 1810: Frequency and Patterns of Opioid Use in the Management of Gout: A Population Based study; Neogi et al.1
The opioid epidemic in the U.S. and Canada has been described as a “one of the worst public health disasters affecting the USA and Canada.”1 Nearly 600,000 people have died from an opioid overdose in the U.S. and Canada over the past 20 years.2 Recommended treatments for gout flares do not include the use of opioids.
Using population-based registry data from Sweden, Neogi et al. report opioid prescribing to be double that of the general population during the first year after a diagnosis of gout (13.1% for patients vs. 6.6% for the general population). Not surprisingly the incidence of opioid prescribing was highest in the first month after the diagnosis. Importantly, the use of medications recommended for gout, such as urate-lowering therapy, non-steroidal anti-inflammatory drugs (NSAIDs) or colchicine was similar or higher in those who received opioids. It is less clear whether combination therapies were used for flares prior to initiation of opioids.
Although it is encouraging to see that recommended medications are used, the use of opioids for patients with gout is concerning. Flare treatments typically are most effective when started early in the course of the flare and at full recommended dose. Once a diagnosis of gout is made people should have ready access to flare treatments. Combination therapies for flares may also be required and should be trialed before opioids.
Additional new, affordable therapies for gout flares are also required.
2. Abstract 0001: 12-Month Findings of the Randomized, Double-Blind, Placebo-Controlled, Multicenter, Efficacy and Safety Study of Methotrexate to Increase Response Rates in Patients with Uncontrolled Gout Receiving Pegloticase (MIRROR RCT); Botson et al.3
The use of pegloticase in the long-term management of gout has been hampered by its immunogenicity, which results in the development of anti-drug antibodies that increase pegloticase clearance and contribute to loss of efficacy, signaled by a rise in serum urate and infusion reactions.
A plenary session during ACR Convergence 2022, included the research by Botson et al. on the 12-month data from the MIRROR RCT. The trial examined the safety and efficacy of 8 mg of pegloticase biweekly in combination with 15 mg of methotrexate weekly vs. placebo. Similar to the previous six-month data from this study, there was lower immunogenicity in the methotrexate group, with only 22.9% of the methotrexate group meeting the serum urate elevation discontinuation criteria, compared with 63.3% of the placebo group.
As one might expect with sustained urate-lowering therapy, additional clinical benefits were evident at week 52, with resolution of tophi occurring in 53.8% of the methotrexate group, compared with 31.0% in the placebo group.
This study provides good evidence for concomitant therapy with methotrexate and pegloticase to reduce the occurrence of immunogenicity and prolong the period before drug withdrawal is necessitated. Given that pegloticase is generally used when all other urate-lowering therapies are ineffective or not tolerated, the loss of efficacy has major clinical implications, and thus, prolongation of its use is clinically important
3–5. Abstract 0112: What Drives Racial Disparities in Gout in the USA?—Population-Based, Sex-Specific, Casual Mediation Analysis; McCormick et al.4
Abstract 1792: Contemporary Racial/Ethnic Disparities in Emergency Department Visits and Hospitalizations for Gout in the United States—2019 Nationwide Analysis; Yokose et al.5
Abstract 1583: Race and Disease Severity Predict Reduced Response to Treat-to-Target Urate Lowering Therapy in Gout: Post-Hoc Analysis of a Multicenter, Randomized, Double-Blind, Non-Inferiority Trial; Helget et al.6
Three important abstracts address racial disparities in gout at this year’s meeting. Data from the STOP gout study (abstract 1583) highlights poor outcomes in non-white participants. Abstract 1792 highlights higher rates of U.S. emergency department visits and hospitalizations in certain ethnic groups, especially Black Americans, with gout. Abstract 0112 attributes much of the difference for the higher frequency of gout in Black women to potentially modifiable clinical factors, such as increased body mass index.
These data are not necessarily novel; rather, it is our response to these data that will be important.
There is a long history of stigmatization and blaming the patient in gout. This is something we cannot and should not accept. The under-representation of minority populations in clinical trials, both industry and investigator led, needs to be addressed. From study inception, through design and recruitment to reporting, we need to consider how to include underserved populations to ensure representation.
Inclusion of investigators and patient research partners of differing ethnicities is an important first step. Systems thinking to address the multiple barriers to healthcare access for under-served populations is required. We need to consider the language we use when reporting our data to ensure the framing of results is appropriate and shifts from blaming the patient.
The consolidated criteria (CONSIDER) statement for strengthening the reporting of health research involving indigenous peoples, published in 2019, is an essential step toward providing guidance for addressing racial disparities, and I encourage you all to read it.7
6. Abstract 1791: Point-of-Care Multi-Energy Photon-Counting CT for Earlier Non-Invasive Diagnosis of Gout and Calcium Pyrophosphate Deposition Disease; Becce et al.8
Although identification of monosodium urate crystals remains the gold standard for the diagnosis of gout, advanced imaging techniques, such as ultrasound and dual-energy computed tomography(DECT), have an increasing role. One of the limitations of DECT is its poor sensitivity to detect the detection of small crystal deposits.
In this abstract, Becce et al. report that point-of-care multi-energy/spectral photon-counting CT (PCCT) is capable of detecting smaller volumes of both monosodium urate and calcium pyrophosphate crystals using soft tissue phantoms.
A non-invasive point-of-care mechanism for assisting with the diagnosis of gout and calcium pyrophosphate deposition disease would be of high clinical use, and I look forward to seeing how this progresses toward clinical use.
7. Abstract 1678: A Genome-Wide Association Analysis of 2,622,830 Individuals Reveals New Pathogenic Pathways in Gout; Merriman et al.9 Note: I am an author on this abstract.
It is well recognized that genetic variants play an important role in regulation of serum urate and development of gout, with the strongest effects observed with genetic variation in the urate transporters in the kidney and gut. The results of this large genome-wide association analysis of 2.6 million people, including 120,000 people with gout, were presented in a plenary session at ACR Convergence 2022. Merriman et al. identified novel loci in this study.
Of particular note, genes in the CHIP (clonal hematopoeiesis of indeterminate potential) were implicated in gout pathogenesis. Further, the novel finding of a genetic association signal at the XDH gene, which encodes the urate-producing enzyme xanthine oxidoreductase (XOR), was observed only in the prostate, suggesting a specific new potential risk factor in men.
These new genetic associations open new lines of inquiry for future research.
 Lisa Stamp, MBChB, PhD, is a rheumatologist, professor of medicine and the associate dean of research at the University of Otago, Christchurch, New Zealand. She has extensively researched the pathophysiology and management of gout.
Lisa Stamp, MBChB, PhD, is a rheumatologist, professor of medicine and the associate dean of research at the University of Otago, Christchurch, New Zealand. She has extensively researched the pathophysiology and management of gout.
Disclosures
Dr. Stamp reports that she is a member of the Pharmaceutical and Therapetuics Advisory Committee for Pharmac New Zealand and has received grant funding from the Health Research Council of New Zealand.
References
- Neogi T, Englund M, Turkiewicz A, Kiadaliri A. Frequency and patterns of opioid use in the management of gout: A population-based study [abstract]. Arthritis Rheumatol. 2022;74(suppl 9).
- The Lancet Public Health. Opioid overdose crisis: Time for a radical rethink. Lancet Public Health. 2022 Mar;7(3):e195.
- Botson J, Saag K, Peterson J, et al. 12-month findings of the randomized, double-blind, placebo-controlled, multicenter, efficacy and safety study of methotrexate to increase response rates in patients with uncontrolled gout receiving pegloticase (MIRROR RCT) [abstract]. Arthritis Rheumatol. 2022;74(suppl 9).
- McCormick N, Lu L, Yokose C, et al. What drives racial disparities in gout in the USA?—Population-based, sex-specific, casual mediation analysis [abstract]. Arthritis Rheumatol. 2022;74(suppl 9).
- Yokose C, McCormick N, Lu N, et al. Contemporary racial/ethnic disparities in emergency department visits and hospitalizations for gout in the United States—2019 nationwide analysis [abstract]. Arthritis Rheumatol. 2022;74(suppl 9).
- Helget L, O’Dell J, Newcomb J, et al. Race and disease severity predict reduced response to treat-to-target urate lowering therapy in gout: Post-hoc analysis of a multicenter, randomized, double-blind, non-inferiority trial [abstract]. Arthritis Rheumatol. 2022;74(suppl 9).
- Huria T, Palmer SC, Pitama S, et al. Consolidating criteria for strengthening reporting of health research involving indigenous peoples: The CONSIDER statement. BMC Med Res Methodol. 2019 Aug 9;19(1):173.
- Becce F, Viry A, Racine D, et al. Point-of-care multi-energy photon-counting CT for earlier non-invasive diagnosis of gout and calcium pyrophosphate deposition disease [abstract]. Arthritis Rheumatol. 2022;74(suppl 9).
- Merriman T, Matsuo H, Takei R, et al. A genome-wide association analysis of 2,622,830 individuals reveals new pathogenic pathways in gout [abstract]. Arthritis Rheumatol. 2022;74(suppl 9).