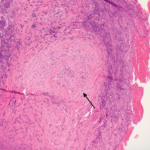
Our patient met the American College of Rheumatology’s 1990 criteria for GPA. He presented with a leukocytoclastic vasculitis of the lung and skin, and pauci-immune necrotizing glomerulonephritis, and was positive for both ANCA and PR3. There were ulcerations on the jejunal biopsy, and focal mild perivascular inflammation was noted in the resected bowel, yet there was no direct, unequivocal evidence of vasculitis. However, the absence of vasculitis findings on bowel biopsy does not rule out GPA. Because the tissue was obtained after the patient received high-dose steroids, plasmapheresis and rituximab, it’s possible the findings of vasculitis were absent because of this immune-suppression therapy. It’s also possible the etiology of the GI manifestations could be linked to other factors. For example, inflammation- and stress-related ulcerations may be slow to heal, especially in patients with renal failure who are receiving concurrent high-dose steroids. Finally, the intractable GI bleeding could be attributed to mucosal sloughing that regressed over time (see Table 1).
In one case series of seven patients with GPA affecting the small intestine and colon, only three had evidence of vasculitis on biopsy.4 The other patients had ulcerations and inflammation on biopsy. All seven patients had renal involvement and the GI involvement occurred early in the course of the disease, as in our patient (see Table 2). Our patient had evidence of ulcerations and inflammation on the biopsies obtained at enteroscopy and in the resected bowel, but there was no vasculitis seen.
Postmortem studies suggest a higher incidence of GI involvement in GPA than reported, with histological evidence of intestinal vasculitis seen in 24% of cases.5 Perhaps the occurrence of intestinal GPA is underestimated due to asymptomatic involvement or due to immunosuppressive treatments masking symptoms. Our patient presented with persistent and recurrent GI bleeding that overshadowed the other vasculitis manifestations.
In the previous case reports, cyclophosphamide was the drug of choice for induction therapy in GPA patients with GI disease.6 However, we administered rituximab and tapered the steroids according to the RAVE protocol. To our knowledge, this is the first case report of successful treatment of GPA-related intestinal disease with rituximab.
The absence of vasculitis findings on bowel biopsy does not rule out GPA.
Conclusion
Our report illustrates the serious effects of GPA on the GI system. It demonstrates that despite a negative biopsy for vasculitis, the GI manifestations can be the source of severe morbidity and mortality. Early evaluation for bleeding or perforation and close collaboration with the gastroenterology and surgical teams are essential to improve patient outcomes.