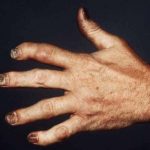
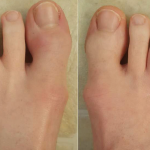
ACR guidelines include recommendations for the management of patients with particular conditions or diseases. Guidelines are developed using a systematic process and are based on available evidence and the clinical experience and expertise of rheumatologists and other interested stakeholders. In the January issue of Arthritis & Rheumatology, Arthritis Care & Research and the Journal of Psoriasis and Psoriatic Arthritis, Singh et al. present the most recent guideline development effort, a joint endeavor from the ACR and the National Psoriasis Foundation, an evidence-based guideline for the pharmacologic and nonpharmacologic treatment of psoriatic arthritis (PsA).
Methods: The authors identified critical outcomes in PsA and clinically relevant PICO (population/intervention/comparator/outcomes) questions. A Literature Review Team performed a systematic literature review to summarize evidence supporting the benefits and harms of available pharmacologic and nonpharmacologic therapies for PsA. GRADE (Grading of Recommendations Assessment, Development and Evaluation) methodology was used to rate the quality of the evidence. A voting panel, including rheumatologists, dermatologists, other health professionals, and patients, achieved consensus on the direction and the strength of the recommendations.
Results: The guideline covers the management of active PsA in patients who are treatment-naive and those who continue to have active PsA despite treatment, and addresses the use of oral small molecules, tumor necrosis factor inhibitors, interleukin-12/23 inhibitors (IL-12/23i), IL-17 inhibitors, CTLA4-Ig (abatacept), and a JAK inhibitor (tofacitinib).
The authors also developed recommendations for psoriatic spondylitis, predominant enthesitis, and treatment in the presence of concomitant inflammatory bowel disease, diabetes, or serious infections. We formulated recommendations for a treat-to-target strategy, vaccinations, and nonpharmacologic therapies. Six percent of the recommendations were strong and 94% conditional, indicating the importance of active discussion between the healthcare provider and the patient to choose the optimal treatment.
Some of the recommendations include:
- Use a TNFi over an oral, small molecule agent (OSM) (i.e., methotrexate) for patients who are treatment naive and have relatively active disease;
- Patients with active PsA despite treatment with an OSM should switch to a TNFi biologic over a different OSM;
- An OSM may be chosen over a TNFi biologic if the patient has mild to moderate disease, has a preference for oral over parenteral therapy or has concerns about the adverse effects of a biologic;
- A TNFi biologic would not be a good choice for those with congestive heart failure, previous serious or recurrent infections or demyelinating disease; and
- For patients who have active PsA and concomitant IBD despite treatment with an OSM:
- Switch to a monoclonal antibody TNFi biologic over a TNFi biologic soluble receptor biologic, which is ineffective for IBD;
- Switch to a TNFi monoclonal antibody biologic over an IL-17i biologic, which is ineffective for IBD; and
- Switch to an IL-12/23i biologic over switching to an IL-17i biologic.
Conclusion: The 2018 ACR/NPF PsA guideline serves as a tool for healthcare providers and patients in the selection of appropriate therapy in common clinical scenarios. Best treatment decisions consider each individual patient situation. The guideline is not meant to be proscriptive and should not be used to limit treatment options for patients with PsA.