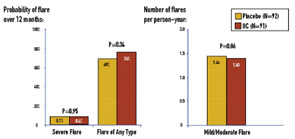
Serious adverse events were rare in the cohort as a whole. There was one serious adverse event—a deep venous thrombosis—among the OC subjects, and three serious adverse events in the placebo group: one ocular thrombosis (after randomization but prior to taking placebo), one superficial thrombophlebitis, and one death a year after study drug (placebo) was discontinued due to severe flare at three months. None of the three subjects who experienced thrombotic adverse events had developed anticardiolipin antibodies.
Another compelling prospective study evaluating the safety of OCs was conducted in Mexico.3 This was a single-blind trial that enrolled 162 women with SLE. Patients were randomized to combined oral contraceptives (30 µg ethinyl estradiol plus 150 µg levonorgestrel), a progestin-only pill (30 µg levonorgestrel), or a copper intra-uterine device (IUD) (TCu 380A, Orthopharmaceuticals), with 54 patients in each treatment group. In this study, the primary outcome was global disease activity, not a severe flare per se. In contrast to SELENA, it was not specifically stated whether women could have received OCs after the diagnosis of SLE for any period of time. Furthermore, patients were not excluded based on titers of anticardiolipin antibodies.
Disease activity remained mild and stable in all groups throughout the course of evaluation. There were no significant differences among the groups during the trial in disease activity, incidence or probability of flares, or medication use. Thromboses occurred in four patients (two in each of the two groups receiving hormones). The authors of this study concluded that global disease activity, maximum SLE Disease Activity Index score, incidence of flares, time to first flare, and incidence of adverse events were similar among patients with SLE, irrespective of the type of contraceptive they received.
The SELENA-HRT study comprised 351 menopausal SLE patients enrolled from 16 university-affiliated rheumatology clinics/practices in 11 U.S. states. Patients were randomized to twelve months of treatment with active drug (0.625 mg conjugated estrogen daily plus an additional pill containing 5 mg medroxyprogesterone for days 1 through 12 of the month) or placebo, and evaluations at months 1, 2, 3, 6, 9, and 12 from the time of enrollment. The occurrence of severe flare was rare in both treatment groups: the 12-month severe-flare rate was 0.081 for HRT and 0.049 for placebo, yielding an estimated difference in severe-flare rates of 0.033 (P=0.23). (See Figure 2) The upper limit of the one-sided 95% CI for the treatment difference was 0.078, which was within the pre-specified margin of 0.09 for non-inferiority.