Physicians caring for women with systemic lupus erythematosus (SLE) often face difficult decisions regarding the use of exogenous estrogens as part of the overall management plan. In certain circumstances the discussion is moot because a patient may have an unambiguous contraindication to estrogen therapy such as a thrombotic diathesis. In other cases, physician and patient must have a lengthy discussion, attempting to balance evidence for and against use. Three recently published randomized controlled trials offer new evidence to aid this discussion.
Strong Evidence from Recent Trials
The Safety of Estrogens in Lupus Erythematosus National Assessment (SELENA) trial comprised two separate studies. (I was the principal investigator on both in partnership with Dr. Michelle Petri.) One study focused on oral contraceptives (OCs) and the other on postmenopausal hormone replacement therapy (HRT).1,2 Both studies were designed as non-inferiority trials to establish that exogenous estrogens were not more likely than placebo to increase the risk of a severe flare.
The rationale for choosing severe flare and not all flares as an endpoint is that for many women a mild flare (e.g., an increase in arthralgias readily treated with over-the-counter nonsteroidal medications) might be an acceptable tradeoff for use of an effective and easy form of birth control. However, because the trial involved a drug that might increase the risk of flare, vigilance with regard to safety mandated that all flares be captured, even those that were extremely mild. Importantly, my colleagues and I excluded patients with a thrombophilic risk, defined specifically as:
- History of deep venous thrombosis, arterial thrombosis, or pulmonary embolus;
- Presence of IgG, IgM, or IgA anticardiolipin antibodies (GPL >40, MPL >40, or APL >50) and/or demonstration of lupus anticoagulant by dilute Russell viper venom time test (dRVVT); and
- History of myocardial infarction.
An important caveat regarding both trials is that patients’ lupus had to be inactive or stable-active for two months prior to randomization.
At first glance, the entry criteria for the trials might suggest that the results would be generalizable only to patients with relatively mild lupus (i.e., without organ-threatening disease). In fact, this was not the case, because approximately 40% of the patients in both trials had a history of lupus nephritis. Many patients had significantly active lupus in the past. While results obtained in the OC trial might not be applicable for the patient with active nephritis about to receive immunosuppressive agents, that same patient, when stabilized for several months, could then have become eligible. The presence of anti-dsDNA antibodies and low levels of complement in nearly a third of the patients again indicated that the results would likely be applicable to the patients seen in the “real world” of lupus.
The results of three large prospective trials indicate that there is generally excellent tolerance of exogenous hormones with regard to disease activity.
Study Specifics and Findings
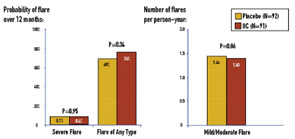
For the SELENA-OC trial, 183 SLE patients were enrolled between June 1997 and July 2002 (with follow-up through July 2003) at 15 U.S. sites. To eliminate bias toward patients who would be likely to do well on OCs, patients were excluded if they had used OCs for more than one month after being diagnosed with SLE. Patients were randomized double-blind to OCs (triphasic 35 µg ethinylestradiol plus 0.5 to 1 mg norethindrone for twelve 28-day cycles) (N=91), or placebo (N=92) and evaluated at months 1, 2, 3, 6, 9, and 12. Thirty-seven percent were Caucasian, 33% African American, 16% Hispanic, and 14% Asian. As detailed in Table 1, the primary endpoint—severe flare—was rare: seven of the 91 patients on OCs (7.7%) versus seven of the 92 patients on placebo (7.6%). The 12-month severe flare rate was 0.084 for OCs and 0.087 for placebo, a difference of -0.0028 (P=0.95). (See Figure 1) The upper limit of the one-sided 95% confidence interval (CI) for this difference was 0.069, within the pre-specified 9% non-inferiority margin. Mild/moderate flare rates were similar: 1.40 versus 1.44 flares/person-years (OCs versus placebo), risk ratio (RR)=0.98, P=0.86.
Serious adverse events were rare in the cohort as a whole. There was one serious adverse event—a deep venous thrombosis—among the OC subjects, and three serious adverse events in the placebo group: one ocular thrombosis (after randomization but prior to taking placebo), one superficial thrombophlebitis, and one death a year after study drug (placebo) was discontinued due to severe flare at three months. None of the three subjects who experienced thrombotic adverse events had developed anticardiolipin antibodies.
Another compelling prospective study evaluating the safety of OCs was conducted in Mexico.3 This was a single-blind trial that enrolled 162 women with SLE. Patients were randomized to combined oral contraceptives (30 µg ethinyl estradiol plus 150 µg levonorgestrel), a progestin-only pill (30 µg levonorgestrel), or a copper intra-uterine device (IUD) (TCu 380A, Orthopharmaceuticals), with 54 patients in each treatment group. In this study, the primary outcome was global disease activity, not a severe flare per se. In contrast to SELENA, it was not specifically stated whether women could have received OCs after the diagnosis of SLE for any period of time. Furthermore, patients were not excluded based on titers of anticardiolipin antibodies.
Disease activity remained mild and stable in all groups throughout the course of evaluation. There were no significant differences among the groups during the trial in disease activity, incidence or probability of flares, or medication use. Thromboses occurred in four patients (two in each of the two groups receiving hormones). The authors of this study concluded that global disease activity, maximum SLE Disease Activity Index score, incidence of flares, time to first flare, and incidence of adverse events were similar among patients with SLE, irrespective of the type of contraceptive they received.
The SELENA-HRT study comprised 351 menopausal SLE patients enrolled from 16 university-affiliated rheumatology clinics/practices in 11 U.S. states. Patients were randomized to twelve months of treatment with active drug (0.625 mg conjugated estrogen daily plus an additional pill containing 5 mg medroxyprogesterone for days 1 through 12 of the month) or placebo, and evaluations at months 1, 2, 3, 6, 9, and 12 from the time of enrollment. The occurrence of severe flare was rare in both treatment groups: the 12-month severe-flare rate was 0.081 for HRT and 0.049 for placebo, yielding an estimated difference in severe-flare rates of 0.033 (P=0.23). (See Figure 2) The upper limit of the one-sided 95% CI for the treatment difference was 0.078, which was within the pre-specified margin of 0.09 for non-inferiority.
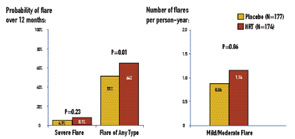
In contrast to severe flares, mild/moderate flares were significantly increased in the HRT arm: 1.14 flares per person-year for HRT and 0.86 for placebo (RR=1.34; P=0.01). The probability of any type of flare by 12 months was 0.64 for HRT and 0.51 for placebo (P=0.01). There was one death in the study, which occurred in the HRT group. There were three deep-vein thromboses (two on HRT and one on placebo), one stroke on HRT, and one thrombosis in an AV graft on HRT.
Mining Retrospective Data and Basic Science
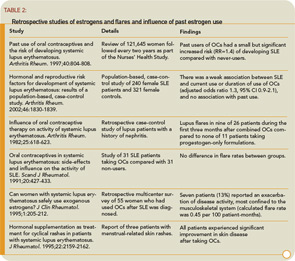
Studies on the influence of past use of estrogens in the development of SLE have not yielded consistent results. (See Table 2) The concern and confusion regarding estrogen effects in SLE have led to studies of estrogen receptor expression in peripheral B cells of SLE patients. Overall, levels of estrogen receptors do not differ between SLE patients and non-autoimmune women. However, a variety of truncated estrogen receptor transcripts that can be produced. One study suggests that a short transcript encoding a constitutively activated estrogen receptor a may be present at higher levels in SLE.4 Murine data suggest that an estrogen-mediated breakdown in B cell tolerance is genetically determined either by polymorphisms of the estrogen receptor genes or, more likely, by polymorphisms of estrogen-inducible genes or of other genes in pathways that involve estrogen-inducible genes.5
Benefits Beyond Birth Control
The clinical relevance of determining the safety of OCs in SLE extends beyond birth control. A well-established salutary effect of estrogen, clearly relevant to women with SLE, is the preventive effect on bone loss and increase in bone mass at the lumbar spine, radius, and hips. Estrogens suppress bone-resorbing cytokines such as interleukin-1 and interleukin-6 and exert positive changes in calcium homeostasis by restoration of a defective synthesis of 1,25–dihydroxyvitamin D and augmentation of intestinal calcium absorption. In a retrospective cohort of 702 women with lupus followed for 5,951 person-years, fractures occurred in 12.3% of the patients, a nearly five-fold increase compared to healthy women.6 In a separate study, osteoporosis was detected in 22.6% of 84 premenopausal SLE patients and appeared to be related to disease duration and use of glucocorticoids.7 Similar observations were noted in a combined (pre- and postmenopausal) cohort of 75 SLE patients, again emphasizing an association with the use of steroids.8 Although prospective studies of OCs have not been performed on glucocorticoid-treated premenopausal women, observational epidemiologic studies suggest that women who received OCs had higher adjusted bone mass density than women who did not.9
The ACR Task Force on Osteoporosis Guidelines recommend OCs to prevent glucocorticoid-induced osteoporosis in premenopausal women with oligo- or amenorrhea. However, an alternative form of contraception, medroxyprogesterone acetate injectable suspension (Depo-Provera), was associated with an increase in osteopenia/osteoporosis after two years of use in a non-SLE female population.10
Cardiovascular Considerations
While there are untoward effects of estrogens on clotting parameters, and data from the prospective Heart and Estrogen/progestin Replacement Study (HERS) and the NIH-sponsored Women’s Health Initiative have not substantiated the expected cardioprotection of hormone replacement in postmenopausal women (even suggested potential risk for coronary heart disease, albeit age from menopause is likely a factor), favorable effects of estrogens on several surrogate markers of cardiac risk may still be relevant to premenopausal women with SLE.11-13 Accumulating data on large numbers of SLE patients strongly support accelerated atherosclerosis, the precise etiology of which remains undefined. It is presently unknown whether OCs confer a beneficial cardiac effect given that estrogens increase HDL cholesterol; decrease both LDL cholesterol and lipoprotein(a); decrease circulating P-selectin, which tethers leukocytes to endothelial cells and activated platelets; and enhance basal nitric oxide production and release. Estradiol reduces the vascular response in an in vitro model of immune injury, the rabbit cardiac allograft, which may mimic the pathogenesis of SLE-accelerated atherosclerosis. Observational studies suggest that higher estrogen status is associated with a decreased mean serum total homocysteine concentration, a risk factor for vascular occlusion.14 Estrogens may have an antioxidant effect, inhibiting peroxidation of vascular smooth muscle membrane phospholipids and thereby peroxidation induced cell growth and migration, an important step in atherosclerosis.
The increased thrombotic risk associated with estrogen may outweigh its protective effects on atherosclerosis in women with established plaque, at least for the first years before longer-term cardioprotective benefits can take effect and have a major impact. Most studies suggest that estrogen inhibits the neointimal response to acute injury in normal blood vessels, but this vasoprotective effect is not seen in vessels with pre-existing atherosclerosis. In contrast, one placebo-controlled study has suggested that short term administration of 17b estradiol is effective in improving effort induced myocardial ischemia in female patients with coronary artery disease.15 Acute administration of conjugated equine estrogens prior to induction of ischemia in dogs attenuated the ventricular arrhythmias of ischemia as well as those of reperfusion.16 In nonhuman primates, OCs do not increase the risk of arterial thrombosis. In fact, there was a reduction in the incidence of thrombosis in the OC-treated animals versus untreated controls.17 Furthermore, OCs inhibited atherosclerosis in a prospective treatment study in 193 adult female cynomolgus monkeys.18
Conclusion
The results of three large prospective trials indicate that there is generally excellent tolerance of exogenous hormones with regard to disease activity. In contrast to the agreement of the two studies on premenopaual patients and OCs with regard to all types of flares, there was a small but significant risk of increasing the natural flare rate of lupus by adding a short course of HRT. Fortunately, the great majority of these flares were mild/moderate.
What is uniformly disconcerting in these trials is the risk of thrombosis, which can certainly erode confidence in choosing OCs or estrogen replacement therapy. This risk must be carefully weighed, and it seems prudent to fully evaluate patients for evidence of thrombophilia before instituting therapy. At the very least, the history of a previous venous or arterial event would be a clear-cut contraindication. The identification of a circulating lupus anticoagulant, anti-cardiolipin, or anti–beta 2 glycoprotein I antibodies of any isotype above a modest range should be reason to dissuade a patient from considering OC or estrogen replacement therapy. While the presence of a false positive Venereal Disease Research Laboratory (VDRL) alone is unusual and likely to be identified by the obstetrician/gynecologist, it too might be reason for seeking alternatives to estrogen use. Given the rapid pace of scientific discovery, we might soon be able to identify which patients might experience flares when exposed to exogenous estrogens.
Dr. Buyon is professor of medicine in the division of rheumatology at New York University School of Medicine in New York.
References:
- Petri M, Kim MY, Kalunian KC, et al. Combined oral contraceptives in women with systemic lupus erythematosus. New Engl J Med. 2005;353:2550-2558.
- Buyon JP, Petri M, Kim MY, et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med. 2005;142:953-962.
- Sanchez-Guerrero J, Uribe AG, Jimenez-Santana L, et al. A trial of contraceptive methods in women with systemic lupus erythematosus. N Engl J Med. 2005;353:2539-2549.
- Liu H, Liu Z, Chen Z, Liu D, Li L. [Estrogen receptor gene polymorphism and its association with clinicopathological manifestation of lupus nephritis.] Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2000;17:266-269.
- Peeva E, Venkatesh J, Michael D, Diamond B. Prolactin as a modulator of B cell function: implications for SLE. Biomed Pharmacother. 2004;58:310-319.
- Ramsey-Goldman R, Dunn JE, Huang CF, et al. Frequency of fractures in women with systemic lupus erythematosus: comparison with United States population data. Arthritis Rheum. 1999;42:882-890.
- Sinigaglia L, Varenna M, Binelli L, et al. Determinants of bone mass in systemic lupus erythematosus: a cross sectional study on premenopausal women. J Rheumatol. 1999;26:1280-1284.
- Gilboe IM, Kvien TK, Haugeberg G, Husby G. Bone mineral density in systemic lupus erythematosus: comparison with rheumatoid arthritis and healthy controls. Ann Rheum Dis. 2000;59:110-115.
- Sowers MFR, Galuska DA. Epidemiology of bone mass in pre-menopausal women. Epidemiol Rev. 1993;15:374-398.
- FDA MedWatch 2004 Safety Alert—Depo-Provera (medroxyprogesterone acetate injectable suspension). www.fda.gov/medwatch/SAFETY/2004/safety04.htm
- Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. J Am Med Assoc. 1998;280:605-613.
- Grady D, Herrington D, Bittner V, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). J Am Med Assoc. 2002;288:49-57.
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. J Am Med Assoc. 2002;288:321-323.
- Morris MS, Jacques FF, Selhub J,Rosenberg IH. Total homocysteine and estrogen status indicators in the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2000;152:140-148.
- Rosano GM, Caixeta AM, Chierchia S, et al. Short term anti ischemic effect of 17beta estradiol in postmenopausal women with coronary artery disease. Circulation. 1997;96:2837 2841.
- McHugh NA, Solowiej A, Klabunde RE, Merrill GF. Acute coronary vascular and myocardial perfusion effects of conjugated equine estrogen in the anesthetized dog. Basic Res Cardiol. 1998;93:470 476.
- Bellinger DA, Williams JK, Adams MR, Honore EK, Bender DE. Oral contraceptives and hormone replacement therapy do not increase the incidence of arterial thrombosis in a nonhuman primate model. Arterioscler Thromb Vasc Biol. 1998;18:92-99.
- Kaplan JR, Adams MR, Anthony MS, Morgan TM, Manuck SB, Clarkson TB. Dominant social status and contraceptive hormone treatment inhibit atherogenesis in premenopausal monkeys. Arterioscler Thromb Vasc Biol. 1995;15:2094-2100.