On Sept. 25, 2013, the Food and Drug Administration (FDA) issued a drug safety communication that described approved modifications to the prescribing information for Rituxan (rituximab) and Arzema (ofatumumab). The changes emphasize the risk of hepatitis B virus (HBV) reactivation with use of these anti-CD20 molecules and provide guidance for screening, monitoring and management of patients to reduce this risk. The revisions were prompted by an FDA review of confirmed HBV reactivation fatalities reported to the Adverse Events Reports database associated with rituximab (31 cases) and ofatumumab (one case).
HBV reactivation is a well-described complication of cytotoxic and immunosuppressive medications, including rituximab, methotrexate, corticosteroids and anti-tumor necrosis factor agents, in patients with prior HBV infection. HBV reactivation begins with increasing serum levels of HBV DNA followed by a rise in serum alanine aminotransferase (ALT) levels. In severe cases, progression to liver failure and death can occur. Given the potential serious consequences of HBV reactivation and the availability of safe and effective HBV antivirals that may prevent HBV reactivation, increased attention to screening and monitoring for HBV in patients receiving immunosuppressive medications is warranted.
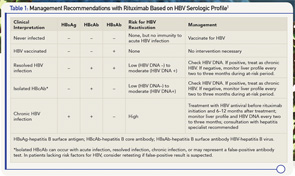
Screen for HBV Before Immunosuppression
The initial screening for HBV consists of HBV surface antigen, HBV core antibody and HBV surface antibody (see Table 1e). Patients who lack all three markers could be considered for vaccination if they have risk factors for HBV. Patients who are HBV surface antigen positive are at high risk for HBV reactivation and should be referred to a hepatitis specialist for antiviral therapy before immunosuppression. Patients with HBV core antibody without HBV surface antigen can still harbor low levels of HBV replication in the serum or liver. In these individuals, the presence of HBV surface antibody reduces, but does not completely eliminate, the risk for HBV reactivation. It is beneficial to test for HBV DNA in all patients who are HBV core antibody positive regardless of HBV surface antibody status. Patients who are HBV DNA positive will likely require prophylaxis with an HBV antiviral before immunosuppression and should be referred to a hepatitis specialist.

Monitor for HBV Reactivation During & After Immunosuppression
Because HBV reactivation can occur at any time during immunosuppression and up to one year after treatment with rituximab, the at-risk period for HBV reactivation extends for 6–12 months after giving each rituximab dose. Patients who are HBV surface antigen positive and/or have detectable levels of HBV DNA should be treated with HBV antivirals a minimum of two or three weeks before beginning rituximab and continued for 6–12 months after therapy with rituximab is completed. Patients should be monitored with a liver profile and HBV DNA every two to three months while on HBV antivirals. The justification for this approach is largely derived from use of rituximab in patients with chronic HBV and malignancy, and the safety of rituximab in patients with chronic HBV infection on antiviral prophylaxis is uncertain.
Patients who are HBV core antibody positive with an undetectable HBV DNA should be serially monitored with liver profile every two to three months during the at-risk period (during therapy and 6–12 months after therapy). Patients with more substantive risks for HBV reactivation (detectable HBV DNA, absence of protective HBsAb levels) may benefit from monitoring with HBV DNA every two to three months, although the cost benefit of this approach has not been established. If an abrupt increase in HBV DNA and/or abnormal liver profile is observed, all immunosuppressive medications should be discontinued and consultation with a hepatitis specialist is advised. There are insufficient data to recommend resumption of rituximab in patients with previous HBV reactivation.

Other Safety Concerns
Given the limited existing data regarding safety of rituximab in rheumatoid arthritis patients receiving treatment for HBV, use of other agents should be considered. Tumor necrosis factor antagonists (TNFs) are contraindicated in Child-Turcotte-Pugh (CTP) Class B-C, but not CTP Class A cirrhosis. HBV antiviral prophylaxis prior to TNF initiation appears to reduce the risk of HBV reactivation in HBV surface antigen-positive patients.2 Provided frequent monitoring for HBV reactivation occurs, TNFs are likely safe to use in CPT Class A HBV surface antigen-positive patients receiving appropriate HBV antivirals.

Treatment of RA in Patients with Chronic Hepatitis C
The risk of hepatitis reactivation appears to be greater among RA patients infected with HBV than those with hepatitis C virus (HCV). Although immunosuppression has been associated with a more rapid progression to HCV-associated cirrhosis, rituximab has been used in HCV-associated cryoglobulinemia with no worsening of HCV-associated liver disease.3 In addition, TNFs appear relatively safe in patients with RA and HCV.4
Mary Jane Burton, MD, is an associate professor of infectious diseases at the University of Mississippi Medical Center. Her research interests include improving outcomes in viral hepatitis. She actively treats hepatitis B and C in her clinics at the Jackson (Miss.) VA Medical Center.
John W. Baddley, MD, MSPH, is professor of medicine in the Division of Infectious Diseases at the University of Alabama at Birmingham. He is also chief of infectious diseases at the Birmingham VA Medical Center.
Kevin L. Winthrop, MD, MPH, is an associate professor of infectious diseases, public health and preventive medicine at Oregon Health and Sciences University in Portland. His research interests include drug safety, and he collaborates closely with rheumatologists in the prevention and treatment of infectious diseases that occur in the setting of biologic therapy.
References
- Winthrop KL, Calabrese LH. Let the fog be lifted: Screening for hepatitis B virus before biological therapy. Ann Rheum Dis. 2011 Oct;70(10):1701–1703.
- Perez-Alvarez R, Diaz-Lagares C, Garcia-Hernandez F, et al. Hepatitis B virus (HBV) reactivation in patients receiving tumor necrosis factor (TNF)-targeted therapy: Analysis of 257 cases. Medicine (Baltimore). 2011 Nov;90(6):359–371.
- Petrarca A, Rigacci L, Caini P, et al. Safety and efficacy of rituximab in patients with hepatitis C virus-related mixed cryoglobulinemia and severe liver disease. Blood. 2010 Jul 22;116(3):335–342.
- Ferri C, Ferraccioli G, Ferrari D, et al. Safety of anti-tumor necrosis factor-alpha therapy in patients with rheumatoid arthritis and chronic hepatitis C virus infection. J Rheumatol. 2008 Oct;35(10):1944–1949.