(click for larger image)
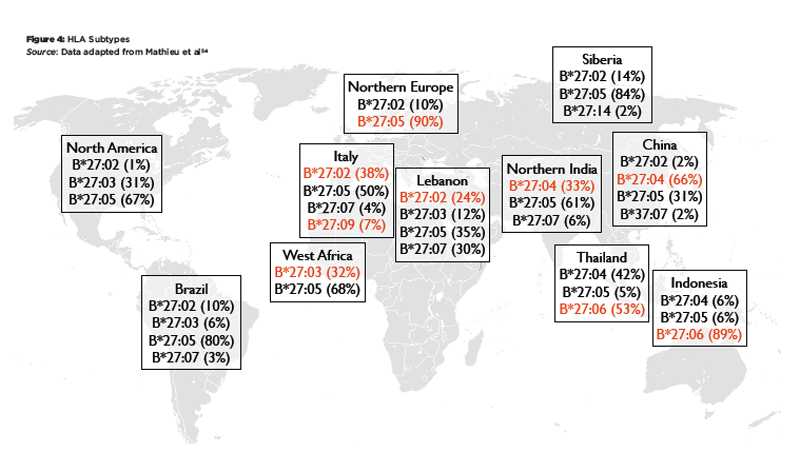
Figure 3: Prevalence of HLA-B27 in Indigenous Populations (%)
Source: Data adapted from Khan14
These patterns reflect two major influences that have shaped MHC profiles in populations: migration and natural selection. For example, HLA-B27 is absent in the indigenous populations of South America, and the HLA-B27 alleles prevalent on this continent today represent genetic admixture from Europe and Africa.15 It has been argued that the lack of HLA-B27 in South America and Southern Africa and the high frequency of HLA-B*27:06 in Southeast Asia may be the result of selective pressure by malaria or other infectious diseases.16 On the other hand, there is evidence that HLA-B27 is associated with HIV elite controller status and higher virus clearance rates after acute HCV infection.17 Thus, being HLA-B27 positive may be a selective advantage in certain circumstances, which might explain the high prevalence in circumpolar regions.
The 2009 NHANES (National Health and Nutrition Examination Survey) study examined the prevalence of axial SpA and HLA-B27 in subjects representative of the U.S. population. Interestingly, HLA-B27 was significantly less frequent in the older age brackets (3.6% in 50–69-year-olds vs. 7.3% in 20–49-year-olds).18 This difference remained statistically significant after adjusting for sex and race/ethnicity, suggesting that HLA-B27 positivity might be associated with early mortality (e.g., via effects on atherosclerosis or cancer), a surprising finding that needs to be confirmed in an independent cohort.
HLA-B27 Transgenic Animals
The debate whether HLA-B27 itself plays a role in the pathogenesis of AS or whether it is simply a genetic marker for another closely linked genetic variant that drives the disease has by now been settled. One important piece of evidence for the direct role of the HLA-B27 molecule was the demonstration that rats transgenic for HLA-B*27:05 and human b2m developed a SpA-like syndrome.19 The disease in HLA-B27 transgenic rats is characterized by colitis and diarrhea, an erosive arthritis affecting peripheral and axial joints, psoriasis-like hyperkeratotic skin lesions and nail dystrophy, as well as orchitis and epididymitis. Some of the phenotypes are strongly microbiota dependent, because germfree rats do not develop arthritis and colitis, but they do develop epididymitis and skin inflammation. Colonization of germfree animals with commensal bacteria reestablished colitis and arthritis.20 Disease could be transferred by transplantation of transgenic bone marrow into non-transgenic recipients, mapping the function of the HLA-B27 transgene to hematopoietically derived cells. However, contrary to expectations based on the canonical function of MHC class I molecules, it has conclusively been shown that CD8+ T cells are dispensable for the development of disease in HLA-B27 transgenic rats.21,22
Hypotheses for the Disease Association of HLA-B27 & AS
Since the discovery of the association between HLA-B27 and AS, many hypotheses have been proposed to explain this association, some of which were short lived. Currently, there are four major hypotheses, which are introduced here. A detailed discussion of this topic and the data supporting or opposing these hypotheses is beyond the scope of this article, and the reader is referred to recent comprehensive reviews on this subject.23-26