(click for larger image)
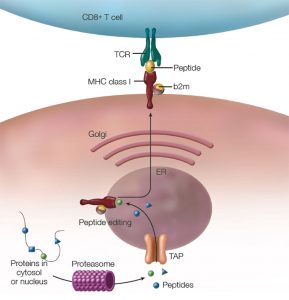
Figure 1: The MHC Class I Antigen Presentation Pathway: TCR = T cell receptor, b2m = beta 2 microglobulin, ER = endoplasmic reticulum, TAP = transporter associated with antigen processing.
Brook Coppola
The mechanistic link between human leukocyte antigen B27 (HLA-B27) and ankylosing spondylitis (AS) is one of the great enigmas in rheumatology. The introduction of biological therapies that target tumor necrosis factor (TNF) or the interleukin (IL) 23/IL-17A axis has had a major impact on the quality of life for many patients with AS, and one might ask: Why should we care about HLA-B27 at all?
Inhibitors of TNF or the IL-23/IL-17A axis are broadly effective in AS. However, they expose patients to the risks of chronic immunosuppression and place a substantial financial burden on the healthcare system. The focus of research, therefore, has to shift toward finding a cure or preventing the disease, which is unlikely to happen without a detailed understanding of the role of HLA-B27 in AS pathogenesis.
This article provides an overview of HLA-B27 research, highlighting important historical landmarks and recent advances.
Discovery of the HLA-B27 Association
In the early 1970s, serological methods originally developed for histocompatibility testing in the organ transplantation field were applied to rheumatic diseases. This resulted in the discovery of the association between HLA-B27 and AS in 1973 by groups in Los Angeles and London.1-3 Subsequently, HLA-B27 was shown to be associated with other types of spondyloarthritis (SpA) or SpA-related disorders as well, namely psoriatic arthritis, reactive arthritis and uveitis.4-7
HLA testing at that time was done with sera from multiparous women, who had become immunized against paternally derived antigenic determinants present on cells of the fetus.
The nature of the antigenic targets remained unknown until the mid-1980s, when the sequences of the HLA genes, including HLA-B27, were determined.8,9 Soon thereafter, it was discovered that the serologically defined HLA-B27 entity was in fact a family of related alleles with minor differences in their amino acid and/or nucleotide sequences and that some of these subtypes were not associated with AS (see below).
MHC Class I Antigen Presentation
HLA-B27 is an allelic variant of the HLA-B gene located in the major histocompatibility complex (MHC) on human chromosome 6p21. The MHC is a highly polymorphic region containing many genes involved in adaptive and innate immunity. HLA-B and the closely related HLA-A and HLA-C genes are located in the class I subregion of the MHC. HLA-A, HLA-B and HLA-C encode the heavy chain (HC) component of MHC class I molecule. MHC class I molecules are heterotrimeric complexes of the highly polymorphic HC, the non-polymorphic beta 2 microglobulin (b2m), which is encoded outside the MHC on chromosome 15, and a peptide of 8–9 amino acid length. The peptide lies in a groove formed by two HC alpha helices on the apical surface of the complex.
MHC class I molecules are expressed on all body cells, where they “present” bound peptides to CD8+ T cells to facilitate peptide-specific cytotoxic T cell responses. MHC class I molecules also interact with a variety of natural killer (NK) cell receptors. This interaction is not peptide specific, although it has been demonstrated that peptide sequences may affect binding strength of some NK cell receptors. MHC class I molecules play critical roles in immune responses against tumors and viruses.
The pathway leading to formation of the HC/b2m/peptide complex (see Figure 1, above) was mapped out in the 1990s:10 Cytosolic proteins (including viral proteins in infected cells or tumor-associated proteins in cancer cells) are degraded by the proteasome. Peptides are then transported from the cytosol into the endoplasmic reticulum (ER) by a peptide pump called TAP (transporter associated with antigen processing). In the ER, these peptides are loaded into the peptide binding groove of nascent MHC class I molecules. This involves the interaction with chaperone proteins that facilitate the correct folding of the HC/b2m/peptide complex. Further, peptidases like ERAP1 (ER aminopeptidase 1) trim the peptides to a length of 8–9 amino acids, which is the optimal size to fit into MHC class I molecules. Once correctly assembled, the HC/b2m/peptide complex moves through the Golgi apparatus to the cell surface.
The amino acid sequence of the MHC class I HC determines which peptides can be loaded and presented on the cell surface (i.e., different MHC alleles present different peptides). The high degree of heterogeneity of HC sequences is thought to represent a safety mechanism that prevents pathogens from escaping an adaptive immune response by mutating their protein sequences so that no pathogen-derived peptide can be presented by any MHC molecule.
MHC Nomenclature & HLA-B27 Variants
The sequences of the polymorphic HLA genes are curated by the WHO Nomenclature Committee for Factors of the HLA System. The current system uses an eight-letter code to categorize each newly discovered MHC class I allele.11 In HLA-B*27:05:02:01, the B refers to the gene locus; the first two digits after the * identify an allele family, which in general corresponds to serological reactivity (e.g., HLA-B27 vs. HLA-B08); subsequent numbers separated by colons group alleles in a hierarchical manner according to differences in amino acid sequence, nucleotide sequence of the coding region and nucleotide sequence of introns/untranslated regions. For example, the protein sequence of HLA-B*27:04 differs from HLA-B*27:05 by three amino acid residues, whereas in HLA-B*27:05:02 and HLA-B*27:05:03 the same amino acid sequence is encoded by a slightly different nucleotide sequence owing to the redundancy of the genetic code.
As of today, more than 100 amino acid coding variants of HLA-B27 have been described. With regard to AS disease association, they fall into three categories:12
- Definitely associated with disease (including 27:02, 27:04, 27:05);
- Not associated with disease and possibly protective (including 27:06, 27:09); or
- Unknown association.
The third group contains the majority of the identified HLA-B27 variants, simply because they are so rare individually that disease association cannot be reliably determined. Sequence analysis suggests that HLA-B*27:05 is the ancestral allele and that the other alleles have evolved from this sequence.13 In the case of the susceptible HLA-B*27:05 and the non-susceptible HLA-B*27:09, the difference is only a single amino acid (amino acid 116 aspartate -> histidine).12
HLA-B27 Assays
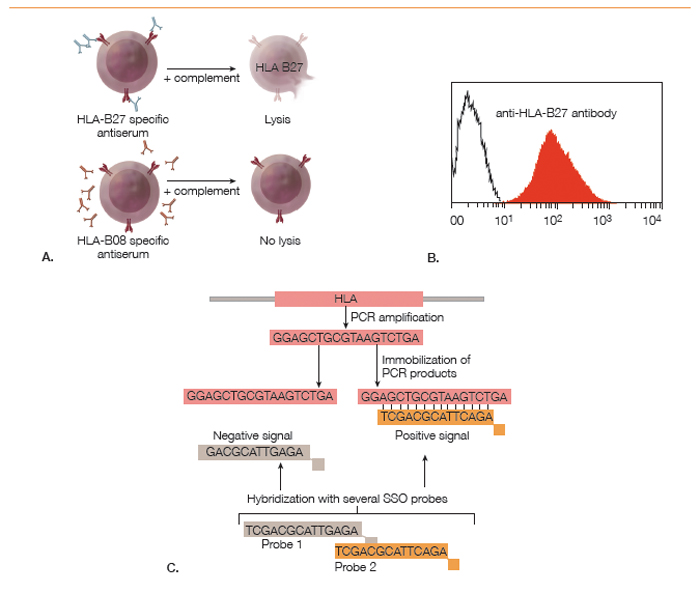
Although HLA-B27 is a genetic marker, the HLA-B27 status of a person can be determined using antibody-based cellular assays or with nucleic acid-based techniques (see Figure 2). The original method for HLA typing, which is still being used by some histocompatibility testing laboratories, was a cytotoxicity assay with monospecific sera from HLA-sensitized individuals and cells from the test subject. The binding of polyclonal anti-HLA-B27 antibodies present in these sera to HLA-B27 proteins on the cell surface results in complement-mediated lysis, which can be read out using a cell viability stain.
(click for larger image)
Figure 2: Methods for Determining HLA-B27 Status: A. Cytotoxicity assay using polyclonal monospecific antisera from sensitized humans; B. Flow cytometry analysis of cells stained with a fluorescently labeled monoclonal anti-HLA-B27 antibody; C. Polymerase chain reaction–sequence specific oligomerization (PCR-SSO). PCR amplification of the polymorphic exon is followed by hybridization with fluorescently labeled oligonucleotide probes to determine subtype identity.
The development of monoclonal antibodies has made HLA-B27 determination by flow cytometry feasible. In this method, peripheral blood cells from the test subject are incubated with a fluorescently labeled monoclonal antibody against HLA-B27. As MHC class I molecules are expressed on all cells, HLA-B27 positivity causes a fluorescence intensity shift of all cells relative to unstained cells or HLA-B27 negative control cells.
Antibody-based cellular techniques can determine only the serotype, which is sufficient in most clinical scenarios. Determining whether a subject is HLA-B*27:05 or HLA-B*27:09 requires a nucleic acid-based method, such as PCR-SSO (polymerase chain reaction–sequence specific oligomerization). This is a two-step method. First, a PCR reaction with common primers flanking exons 2 and 3 of the HLA-B gene is performed to amplify the polymorphic regions of the HC. In the second step, the PCR product is hybridized with a series of sequence-specific labeled oligonucleotides, which bind only in case of sequence complementarity. The hybridization pattern can then be used to infer the identity of the HLA allele. Alternatively, the nucleotide sequence of the PCR fragment is directly determined by DNA sequencing.
Worldwide Distribution of HLA-B27 Alleles
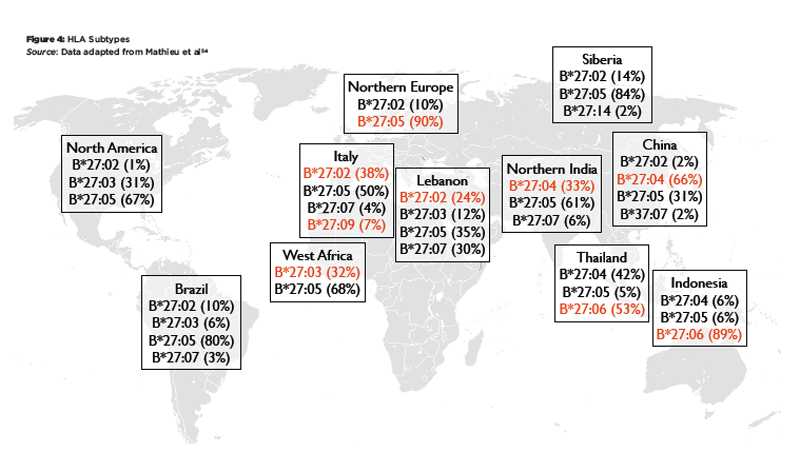
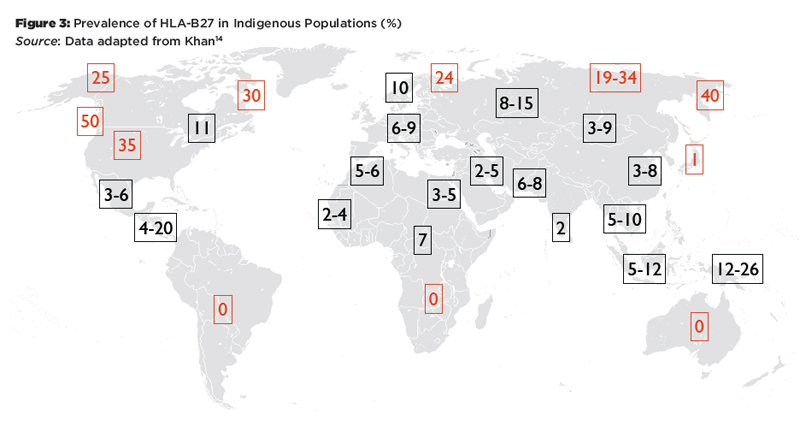
There are striking differences in the worldwide distribution of HLA-B27 and its variants (see Figure 3 and Figure 4). HLA-B27 is very rare in the southern hemisphere and also in Japan.14 It is generally much more common in the northern hemisphere, particularly in indigenous populations of the circumpolar regions. The HLA-B27 variants also show marked differences in their worldwide distribution. HLA-B*27:05 is found in central Europe, HLA-B*27:02 is common in the Mediterranean, and HLA-B*27:04 is the predominant allele in China. Among the non-associated alleles, HLA-B*27:06 is dominant in Southeast Asia, and HLA-B*27:09 is found on the Mediterranean island of Sardinia.
(click for larger image)
Figure 3: Prevalence of HLA-B27 in Indigenous Populations (%)
Source: Data adapted from Khan14
These patterns reflect two major influences that have shaped MHC profiles in populations: migration and natural selection. For example, HLA-B27 is absent in the indigenous populations of South America, and the HLA-B27 alleles prevalent on this continent today represent genetic admixture from Europe and Africa.15 It has been argued that the lack of HLA-B27 in South America and Southern Africa and the high frequency of HLA-B*27:06 in Southeast Asia may be the result of selective pressure by malaria or other infectious diseases.16 On the other hand, there is evidence that HLA-B27 is associated with HIV elite controller status and higher virus clearance rates after acute HCV infection.17 Thus, being HLA-B27 positive may be a selective advantage in certain circumstances, which might explain the high prevalence in circumpolar regions.
The 2009 NHANES (National Health and Nutrition Examination Survey) study examined the prevalence of axial SpA and HLA-B27 in subjects representative of the U.S. population. Interestingly, HLA-B27 was significantly less frequent in the older age brackets (3.6% in 50–69-year-olds vs. 7.3% in 20–49-year-olds).18 This difference remained statistically significant after adjusting for sex and race/ethnicity, suggesting that HLA-B27 positivity might be associated with early mortality (e.g., via effects on atherosclerosis or cancer), a surprising finding that needs to be confirmed in an independent cohort.
HLA-B27 Transgenic Animals
The debate whether HLA-B27 itself plays a role in the pathogenesis of AS or whether it is simply a genetic marker for another closely linked genetic variant that drives the disease has by now been settled. One important piece of evidence for the direct role of the HLA-B27 molecule was the demonstration that rats transgenic for HLA-B*27:05 and human b2m developed a SpA-like syndrome.19 The disease in HLA-B27 transgenic rats is characterized by colitis and diarrhea, an erosive arthritis affecting peripheral and axial joints, psoriasis-like hyperkeratotic skin lesions and nail dystrophy, as well as orchitis and epididymitis. Some of the phenotypes are strongly microbiota dependent, because germfree rats do not develop arthritis and colitis, but they do develop epididymitis and skin inflammation. Colonization of germfree animals with commensal bacteria reestablished colitis and arthritis.20 Disease could be transferred by transplantation of transgenic bone marrow into non-transgenic recipients, mapping the function of the HLA-B27 transgene to hematopoietically derived cells. However, contrary to expectations based on the canonical function of MHC class I molecules, it has conclusively been shown that CD8+ T cells are dispensable for the development of disease in HLA-B27 transgenic rats.21,22
Hypotheses for the Disease Association of HLA-B27 & AS
Since the discovery of the association between HLA-B27 and AS, many hypotheses have been proposed to explain this association, some of which were short lived. Currently, there are four major hypotheses, which are introduced here. A detailed discussion of this topic and the data supporting or opposing these hypotheses is beyond the scope of this article, and the reader is referred to recent comprehensive reviews on this subject.23-26
1) Arthritogenic/spondylogenic peptide hypothesis: This hypothesis is based on the canonical function of HLA-B27 as an MHC class I molecule presenting antigenic peptides to CD8+ T cells. Disease is thought to be the result of a T cell-mediated anti-self-immune response against particular HLA-B27–peptide combinations.27 The peptide hypothesis has an advantage in that it can explain the organ specificity of AS by assuming that expression of the arthritogenic/spondylogenic peptides is restricted to the affected tissues. The model implies breakdown of tolerance toward self-peptides, which may occur in the context of an infection and may involve cross-reactivity with microbial peptides. However, no specific arthritogenic/spondylogenic peptides have been identified so far, and there is no convincing evidence for a critical role for CD8+ T cells in AS pathogenesis.
2) Misfolding hypothesis: Shortly after the demonstration that MHC class I HCs require the interaction with b2m for proper folding in the ER, it was found that HLA-B27 is special in that HLA-B27 HCs have an increased propensity to misfold, even in the presence of b2m.28,29 The accumulation of misfolded proteins in the ER is known to trigger a cellular stress response called unfolded protein response (UPR). The UPR involves multiple pathways all directed at resolving the accumulation of misfolded proteins in the ER. However, in certain situations, UPR activation can induce an inflammatory response.30 This has been demonstrated in cells from HLA-B27 transgenic rats, thereby offering a plausible explanation for the development of disease in these animals without a requirement for CD8+ T cells.31,32 However, there is little supportive evidence for aberrant activation of the UPR in HLA-B27+ humans with AS. (Editor’s note: For more on this hypothesis, see “Conformational Flexibility” on p. 20 in this issue.)
3) Homodimer/NK cell receptor hypothesis: Around the same time that misfolding of HLA-B27 HCs was described, another special molecular feature of HLA-B27 was discovered. HLA-B27 HCs may form disulfide-linked HC homodimers, which do not require b2m for cell surface expression. It was subsequently shown that the inhibitory NK cell receptor KIR3DL2 interacts more strongly with HLA-B27 HC homodimers than with conventional MHC class I complexes.33 Despite their name, the expression of many NK cell receptors, including KIR3DL2, is not limited to NK cells, and there are data demonstrating that HLA-B27 homodimers may result in inappropriate activation of KIR3DL2 expressing CD4+ T cells.34 Why ligation of an inhibitory NK cell receptor would drive CD4+ T cell activation and how such non-antigen-specific T cell activation would lead to a distinctly organ-specific disease are some of the questions that remain to be answered.
4) Microbial hypotheses: Multiple lines of evidence suggest the involvement of microbes in the pathogenesis of HLA-B27 dependent disease. This includes the association of HLA-B27 with reactive arthritis triggered by certain Gram-negative bacteria, the high prevalence of subclinical intestinal inflammation in AS patients and the microbiota dependence of the arthritis in HLA-B27 transgenic rats described earlier.35,36 Potential mechanisms to explain these associations include immunological cross-reactivity with microbial antigens, dysregulated defenses against intracellular bacteria in HLA-B27 expressing phagocytes or indirect effects via the intestinal microbiome.37
New Genetic Data on HLA-B27 & AS
The association of MHC variants with AS was recently revisited using Immunochip data from 9,069 AS cases and 13,578 population controls.38 In this study, the sequences of the MHC genes were imputed (i.e., they were inferred based on the presence of certain local SNPs rather than determined through nucleotide-by-nucleotide sequencing). Genomic sequence imputation is possible because of linkage disequilibrium, which means that two neighboring genetic loci are likely to be inherited in a linked fashion. Therefore, by knowing the sequence (or the SNP pattern) at one locus, one also knows (with a certain probability) the sequence of the closely linked locus.
Cortes et al found, not surprisingly, that HLA-B*27:02 and HLA-B*27:05 were very strongly associated with AS. After conditioning on these two variants, they identified additional associations with non-HLA-B27 variants (i.e., HLA-B*51:01, HLA-B*47:01, HLA-B*40:02, HLA-B*13:02 and HLA-B*40:01), confirming earlier reports of additional MHC class I associations with AS. HLA-B*07:02 and HLA-B*57:01 had small protective effects. Because the study population was exclusively European, the effects of other HLA-B alleles (e.g., 27:03, 27:06, 27:09) could not be ascertained.
Cortes et al also analyzed the association of the different MHC alleles with disease at the amino acid level and found an extremely strong association with amino acid 97 (p<10-3221). This amino acid is located at the bottom of the peptide binding groove, near the so-called F pocket, where the C terminus of the peptide binds. Asparagine or threonine residues at position 97 were associated with AS, whereas serine decreased disease risk. Although the association with amino acid 97 could reflect linkage disequilibrium with the real disease-causing variants, it’s important to note that amino acid 97 is in immediate proximity to amino acid 116, a position that critically determines disease susceptibility. The AS-associated HLA-B*27:05 has aspartic acid in position 116, whereas the non-associated and otherwise sequence-identical HLA-B*27:09 allele has a histidine at position 116.
Genetic Interaction of HLA-B27 & ERAP1/2
Despite the strong association between HLA-B27 and AS, this gene has been estimated to explain only 40% of the genetic variance. Genome-wide association studies (GWAS) have revealed additional genetic associations with AS. Most relevant for this discussion, polymorphisms in ERAP1, ERAP2 and other peptidases involved in processing peptides for presentation in the MHC class I pathway were identified. ERAP1 and ERAP2 are ER-associated aminopeptidases (i.e., enzymes that trim peptides from the amino terminus prior to loading into nascent MHC molecules). ERAP1 is highly polymorphic, with many of these polymorphisms resulting in amino acid changes that affect enzyme activity.
Importantly, the association of ERAP1 with AS susceptibility is present only in HLA-B27-positive individuals (i.e., there is an epistatic interaction between the polymorphisms in the HLA-B and ERAP1 genes with regard to AS susceptibility). Similar interactions have been described for psoriasis (HLA-C*06:02 and ERAP1), Behçet’s disease (HLA-B*51 and ERAP1) and birdshot retinopathy, an inflammatory disease of the eye (HLA-A*29 and ERAP2).39-41 McGonagle has termed these diseases MHC I-opathies.38 Whether the mechanisms linking these MHC class I/ERAP pairs and disease are the same or different needs to be investigated.
HLA-B27 in Clinical Practice
HLA-B27 is included as a parameter in the Assessment of Spondyloarthritis International Society (ASAS) classification criteria for axial (see Figure 5) and peripheral SpA.43,44 However, HLA-B27 is not included in the modified New York criteria (see Figure 6), which are frequently used to diagnose AS, and HLA-B27 positivity is neither required nor sufficient for making a diagnosis of AS or SpA.45
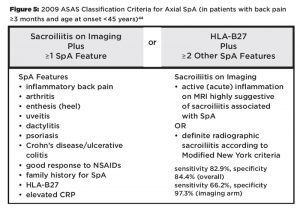
(click for larger image)
Figure 5: 2009 ASAS Classification Criteria for Axial SpA (in patients with back pain ≥3 months and age at onset44
Data regarding the prognostic or predictive value of HLA-B27 are heterogeneous. A recent analysis of genetic factors affecting radiographic disease severity in a cohort of 1,537 AS patients with established disease (mean disease duration ~20 years) failed to show a correlation between HLA-B27 status and radiographic disease severity in the spine.46 In contrast, HLA-B27 positivity was associated with more rapid radiographic progression in the SI joints in a cohort of patients with early axial SpA.47 HLA-B27 positivity was identified as a predictor of a good response to TNF inhibitor therapy in two studies, but not in others, possibly because the latter studies were small and did not include enough HLA-B27 negative patients.48-50 It is unclear whether the difference in response to TNF inhibition was due to an earlier diagnosis in HLA-B27+ subjects or whether there is a true difference in biology.49,51
The picture becomes even more complex when SpA is considered more broadly. A recent study from Dublin analyzed MHC class I genotypes and SpA disease manifestations in a clinically well-characterized cohort of 282 psoriatic arthritis patients.52,53 This study reproduced the well-described associations of HLA-C*06:02 with psoriasis and HLA-B*27:05 with axial disease, in particular with symmetric sacroiliitis. Importantly, additional associations between other MHC class I alleles and distinct disease phenotypes were identified, including an association of HLA-B*08:01 with asymmetric sacroiliitis, and HLA-C*02:02 with arthritis mutilans. Thus, although there is currently no added value in measuring HLA-B27 in patients with a clinical diagnosis of AS and positive radiographs of the SI joints, HLA-B27 and/or comprehensive MHC class I genotyping may have utility, pending confirmation in independent studies, to identify relevant SpA subsets and predict therapeutic responsiveness in the future.
Conclusion
Recent genetic data suggest that AS may be only one example of a group of diseases characterized by epistatic interaction between MHC class I alleles and enzyme variants that affect peptide loading in the ER (MHC I-opathies). The genetic interaction between HLA-B27 and ERAP1 underscores the importance of the MHC class I peptide presentation pathway in the pathogenesis of AS. It favors but does not prove that the arthritogenic/spondylogenic peptide hypothesis is correct because peptide loading will also affect the folding and stability of MHC class I molecules and the specificity of antimicrobial immune responses.
Investigating more broadly the relationship between distinct SpA phenotypes and the various predisposing MHC class I alleles may hold the key to identifying the mechanism underlying the association between HLA-B27 and AS, which has been chased by two generations of researchers since its description in 1973.
 Joerg Ermann, MD, is a rheumatologist in the Division of Rheumatology, Immunology, and Allergy at Brigham and Women’s Hospital and Instructor in Medicine at Harvard Medical School. His clinical and research interests are in spondyloarthritis. His lab studies disease mechanisms relevant to spondyloarthritis using mouse models and translational approaches.
Joerg Ermann, MD, is a rheumatologist in the Division of Rheumatology, Immunology, and Allergy at Brigham and Women’s Hospital and Instructor in Medicine at Harvard Medical School. His clinical and research interests are in spondyloarthritis. His lab studies disease mechanisms relevant to spondyloarthritis using mouse models and translational approaches.
References
- Schlosstein L, Terasaki PI, Bluestone R, et al. High association of an HL-A antigen, W27, with ankylosing spondylitis. N Engl J Med. 1973 Apr 5;288(14):704–706.
- Caffrey MF, James DC. Human lymphocyte antigen association in ankylosing spondylitis. Nature. 1973 Mar 9;242(5393):121.
- Brewerton DA, Hart FD, Nicholls A, et al. Ankylosing spondylitis and HL-A 27. Lancet. 1973 Apr 28;1(7809):904–907.
- Brewerton DA, Caffrey M, Nicholls A, et al. HL-A 27 and arthropathies associated with ulcerative colitis and psoriasis. Lancet. 1974 May 18;1(7864):956–958.
- Aho K, Ahvonen P, Lassus A, et al. HL-A antigen 27 and reactive arthritis. Lancet. 1973 Jul 21;2(7821):157.
- Brewerton DA, Caffrey M, Nicholls A, et al. Reiter’s disease and HL-A 27. Lancet. 1973, Nov 3;302(7836):996–998.
- Brewerton DA, Caffrey M, Nicholls A, et al. Acute anterior uveitis and HL-A 27. Lancet. 1973, Nov 3;302(7836):994–996.
- Ezquerra A, Bragado R, Vega MA, et al. Primary structure of papain-solubilized human histocompatibility antigen HLA-B27. Biochemistry. 1985 Mar 26;24(7):1733–1741.
- Szöts H, Riethmüller G, Weiss E, et al. Complete sequence of HLA-B27 cDNA identified through the characterization of structural markers unique to the HLA-A, -B, and -C allelic series. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1428–1432.
- Neefjes J, Jongsma ML, Paul P, et al. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol. 2011 Nov;11(12):823–836.
- Marsh SG, Albert ED, Bodmer WF, et al. Nomenclature for factors of the HLA system, 2010. Tissue Antigens. 2010 Apr;75(4):291–455.
- Reveille JD. Major histocompatibility genes and ankylosing spondylitis. Best Pract Res Clin Rheumatol. 2006 Jun;20(3):601–609.
- López de Castro JA. HLA-B27 and HLA-A2 subtypes: Structure, evolution and function. Immunol Today. 1989 Jul;10(7):239–246.
- Khan MA. HLA-B27 and its subtypes in world populations. Curr Opin Rheumatol. 1995 Jul;7(4):263–269.
- López-Larrea C, Gonzalez-Roces S, Peña M, et al. Characterization of B27 haplotypes by oligotyping and genomic sequencing in the Mexican Mestizo population with ankylosing spondylitis: Juvenile and adult onset. Hum Immunol. 1995 Jul;43(3):174–180.
- Mathieu A, Cauli A, Fiorillo MT, et al. HLA-B27 and ankylosing spondylitis geographic distribution versus malaria endemic: Casual or causal liaison? Ann Rheum Dis. 2008 Jan;67(1):138–140.
- Neumann-Haefelin C. HLA-B27-mediated protection in HIV and hepatitis C virus infection and pathogenesis in spondyloarthritis: Two sides of the same coin? Curr Opin Rheumatol. 2013 Jul;25(4):426–433.
- Reveille JD, Hirsch R, Dillon CF, et al. The prevalence of HLA-B27 in the United States: Data from the U.S. National Health and Nutrition Examination Survey, 2009. Arthritis Rheum. 2012 May;64(5):1407–1411.
- Hammer RE, Maika SD, Richardson JA, et al. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human beta 2m: An animal model of HLA-B27-associated human disorders. Cell. 1990 Nov 30;63(5):1099–1112.
- Rath HC, Herfarth HH, Ikeda JS, et al. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest. 1996 Aug 15;98(4):945–953.
- May E, Dorris ML, Satumtira N, et al. CD8 alpha beta T cells are not essential to the pathogenesis of arthritis or colitis in HLA-B27 transgenic rats. J Immunol. 2003 Jan 15;170(2):1099–1105.
- Taurog JD, Dorris ML, Satumtira N, et al. Spondylarthritis in HLA-B27/human beta2-microglobulin-transgenic rats is not prevented by lack of CD8. Arthritis Rheum. 2009 Jul;60(7):1977–1984.
- Bowness P. HLA-B27. Annu Rev Immunol. 2015;33:29–48.
- Sorrentino R, Böckmann RA, Fiorillo MT. HLA-B27 and antigen presentation: At the crossroads between immune defense and autoimmunity. Mol Immunol. 2014 Jan;57(1):22–27.
- Colbert RA, Tran TM, Layh-Schmitt G. HLA-B27 misfolding and ankylosing spondylitis. Mol Immunol. 2014 Jan;57(1):44–51.
- Asquith M, Elewaut D, Lin P, et al. The role of the gut and microbes in the pathogenesis of spondyloarthritis. Best Pract Res Clin Rheumatol. 2014 Oct;28(5):687–702.
- Benjamin R, Parham P. Guilt by association: HLA-B27 and ankylosing spondylitis. Immunol Today. 1990 Apr;11(4):137–142.
- Hughes EA, Hammond C, Cresswell P. Misfolded major histocompatibility complex class I heavy chains are translocated into the cytoplasm and degraded by the proteasome. Proc Natl Acad Sci U S A. 1997 Mar 4;94(5):1896–1901.
- Mear JP, Schreiber KL, Munz C, et al. Misfolding of HLA-B27 as a result of its B pocket suggests a novel mechanism for its role in susceptibility to spondyloarthropathies. J Immunol. 1999 Dec 15;163(12):6665–6670.
- Bettigole SE, Glimcher LH. Endoplasmic reticulum stress in immunity. Annu Rev Immunol. 2015;33:107–138.
- Smith JA, Barnes MD, Hong D, et al. Gene expression analysis of macrophages derived from ankylosing spondylitis patients reveals interferon-gamma dysregulation. Arthritis Rheum. 2008 Jun;58(6):1640–1649.
- Delay ML, Turner MJ, Klenk EI, et al. HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum. 2009 Aug 27;60(9):2633–2643.
- Wong-Baeza I, Ridley A, Shaw J, et al. KIR3DL2 binds to HLA-B27 dimers and free H chains more strongly than other HLA class I and promotes the expansion of T cells in ankylosing spondylitis. J Immunol. 2013 Apr 1;190(7):3216–3224.
- Ridley A, Hatano H, Wong-Baeza I, et al. Activation-induced killer cell immunoglobulin-like receptor 3DL2 binding to HLA-B27 licenses pathogenic T cell differentiation in spondyloarthritis. Arthritis Rheumatol. 2016 Apr;68(4):901–914.
- Carter JD, Hudson AP. Reactive arthritis: Clinical aspects and medical management. Rheum Dis Clin North Am. 2009 Feb;35(1):21–44.
- Van Praet L, Jacques P, Van den Bosch F, et al. The transition of acute to chronic bowel inflammation in spondyloarthritis. Nat Rev Rheumatol. 2012;8(5):288–295.
- Lin P, Bach M, Asquith M, et al. HLA-B27 and human β2-microglobulin affect the gut microbiota of transgenic rats. PLoS One. 2014;9(8):e105684.
- Cortes A, Pulit SL, Leo PJ, et al. Major histocompatibility complex associations of ankylosing spondylitis are complex and involve further epistasis with ERAP1. Nat Commun. 2015;6:7146.
- Strange A, Capon F, Spencer CC, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet. 2010 Nov;42(11):985–990.
- Kirino Y, Bertsias G, Ishigatsubo Y, et al. Genome-wide association analysis identifies new susceptibility loci for Behçet’s disease and epistasis between HLA-B*51 and ERAP1. Nat Genet. 2013 Feb;45(2):202–207.
- Kuiper JJ, Van Setten J, Ripke S, et al. A genome-wide association study identifies a functional ERAP2 haplotype associated with birdshot chorioretinopathy. Hum Mol Genet. 2014 Nov 15;23(22):6081–6087.
- McGonagle D, Aydin SZ, Gül A, et al. ‘MHC-I-opathy’-unified concept for spondyloarthritis and Behçet disease. Nat Rev Rheumatol. 2015 Dec;11(12):731–740.
- Rudwaleit M, van der Heijde D, Landewé R, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): Validation and final selection. Ann Rheum Dis. 2009 Jun;68(6):777–783.
- Rudwaleit M, van der Heijde D, Landewé R, et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. 2011 Jan;70(1):25–31.
- van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984 Apr;27(4):361–368.
- Cortes A, Maksymowych WP, Wordsworth BP, et al. Association study of genes related to bone formation and resorption and the extent of radiographic change in ankylosing spondylitis. Ann Rheum Dis. 2015 Jul;74(7):1387–1393.
- Dougados M, Demattei C, van den Berg R, et al. Rate and predisposing factors of sacroiliac radiographic progression after a 2 years follow-up period in recent onset spondyloarthritis. Arthritis Rheumatol. 2016 Mar 18.
- Rudwaleit M, Claudepierre P, Wordsworth P, et al. Effectiveness, safety, and predictors of good clinical response in 1250 patients treated with adalimumab for active ankylosing spondylitis. J Rheumatol. 2009 Apr;36(4):801–808.
- Vastesaeger N, van der Heijde D, Inman RD, et al. Predicting the outcome of ankylosing spondylitis therapy. Ann Rheum Dis. 2011 Jun;70(6):973–981.
- Arends S, van der Veer E, Kallenberg CG, et al. Baseline predictors of response to TNF-α blocking therapy in ankylosing spondylitis. Curr Opin Rheumatol. 2012 May;24(3):290–298.
- Chung HY, Machado P, van der Heijde D, et al. HLA-B27 positive patients differ from HLA-B27 negative patients in clinical presentation and imaging: Results from the DESIR cohort of patients with recent onset axial spondyloarthritis. Ann Rheum Dis. 2011 Nov;70(11):1930–1936.
- Winchester R, Minevich G, Steshenko V, et al. HLA associations reveal genetic heterogeneity in psoriatic arthritis and in the psoriasis phenotype. Arthritis Rheum. 2012 Apr;64(4):1134-1144.
- Haroon M, Winchester R, Giles JT, et al. Certain class I HLA alleles and haplotypes implicated in susceptibility play a role in determining specific features of the psoriatic arthritis phenotype. Ann Rheum Dis. 2016 Jan;75(1):155–162.
- Mathieu A, Paladini F, Vacca A, et al. The interplay between the geographic distribution of HLA-B27 alleles and their role in infectious and autoimmune diseases: A unifying hypothesis. Autoimmun Rev. 2009 Mar;8(5):420–425.