HMGB1 can serve as an alarmin. Alarmins are cellular molecules that promote inflammation and activate innate and adaptive immunity. In its activity as an alarmin, HMGB1 can interact with receptors that include receptor for advanced glycation end products (RAGE), and Toll-like receptors (TLR) 2, 4, and 9. There is increasing evidence that HMGB1 is involved in autoimmunity and chronic inflammation, as exemplified by increased levels of HMGB1 in the sera of patients with rheumatic disease. Recently, an association between the presence of HMGB1 and chronic renal diseases has been established, suggesting this protein has a role as a biomarker for assessing kidney outcome. In this review, we will discuss the biological properties of HMGB1 and summarize the recent advances on its role in inflammatory and chronic renal disease, including systemic lupus erythematosus.
Introduction
HMGB1 is a nonhistone nuclear protein that has attracted great interest as a protype for a dual-function molecule. Indeed, as shown in provocative in vitro and in vivo studies, HMGB1 has highly diverse biological properties that extend from DNA binding to transcriptional regulation to chromatin architecture and, most surprisingly, to immune activation. The study of this protein is providing an important new perspective on the biology of nuclear molecules and is illuminating the diversity of protein utilization in immune regulation. Furthermore, the study of HMGB1 is establishing the foundation for new approaches to monitor renal disease and develop novel immunosuppressive therapy.
HMGB1, which has a molecular mass of 30kD, is found in all mammalian tissues and is highly conserved in sequence among species. HMGB1 resides primarily in the cell nucleus but can be found in cytoplasm in certain types of cell. While the original literature on HMGB1 focused on its activity in the cell nucleus, a series of remarkable studies showed that HMGB1 can translocate to the outside of cells and, in the extracellular space, can serve as an alarmin. Alarmins are endogenous molecules, both large and small, that have the ability to alert the innate and adaptive immune system.1 HMGB1 is a prototypic alarmin that, once released into the extracellular space, can act as an inflammatory mediator in many rheumatic diseases, including systemic lupus erythematosus (SLE).2,3 This review will summarize the biological features of HMGB1 and its role as an inflammatory mediator in chronic kidney diseases. Moreover, this review will consider the role of HMGB1 in the pathogenesis of nephritis in SLE
HMGB1: Eternity Molecule with Many Functions
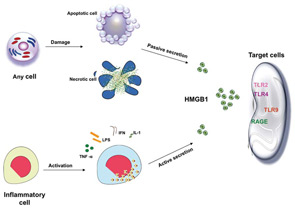
HMGB1 is a ubiquitous protein that is found in almost every type of cell in the body; however, not every cell is able to secrete HMGB1. Although HMGB1 secretion from immune cells has been widely described in literature, data from a variety of experimental systems indicate that that it can be also released from nonimmune cells. What is most surprising and intriguing about HMGB1 is that it has double life. Thus, HMGB1 has important biological functions inside as well as outside the cell, displaying highly distinct activities that encompass immune stimulation. This double life is based on the secretion and release of HMGB1 into the extracellular space during cellular activation and cellular death. While HMGB1 is actively secreted from activated immune-inflammatory cells such as monocytes and macrophages following stimulation with proinflammatory cytokines (tumor necrosis factor [TNF]-α, interleukin [IL]-1, interferon [IFN]-γ) and bacterial products (lipopolysaccharide [LPS]), its release can also occur passively during necrosis as well as during the late phase of apoptosis (see Figure 1).4,5
Recent reports have highlighted the importance of the intracellular distribution of HMGB1 in determining its potential biological activity. Inside the cell, in the nucleus, HMGB1 binds to DNA and modulates a variety of processes including the determination of chromosomal architecture and transcriptional regulation. Once translocated from the nucleus and released outside the cell, HMGB1 can function as an inflammatory mediator contributing to many inflammatory processes.6 An important question raised about role of HMGB1 in inflammation relates to whether it can promote inflammation on its own or must operate in tandem with another conventional or nonconventional proinflammatory mediator. Indeed, compelling data indicate that HMGB1 can exert its role as alarmin only when complexed with proinflammatory molecules, endogenous and exogenous substances such as cytokines or TLR ligands.3
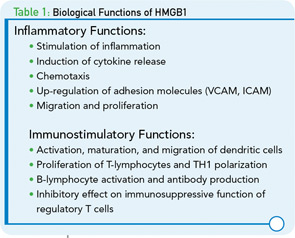
In addition to its role in innate immunity, HMGB1 can also modulate events in adaptive immune responses. As shown in a recent study, HMGB1 possesses immunostimulatory properties and can activate dendritic cells (DCs), T-lymphocytes, and B-lymphocytes. Further, data indicate that HMGB1 can mediate the maturation of DCs and their migration of DCs, with DCs both secreting and responding to HMGB1.7 In addition, HMGB1 has an important role in T-cell survival, and enables proliferation and polarization of naive CD4 T-lymphoyctes towards T-helper 1 phenotype (Th-1).8
Other intriguing studies have focused on the effect of HMGB1 on regulatory T cells. Regulatory T cells have important functions in maintaining immunological tolerance, with disturbances in their number or activity predisposing to inflammatory and autoimmune diseases. In animal models, HMGB1 released after injury showed an inhibitory effect on the immunosuppressive function of T-regulatory cells.9 Whether HMGB1 influences the immunosuppressive function of regulatory T cells in the human situation requires further study. Another facet of HMGB1 activity relevant to autoimmunity concerns the ability of this protein to activate autoreactive B-cells and promote their responses to endogenous substances.10 Functions of HMGB1 are summarized in Table 1.
Mode of Action
To function as an alarmin, nuclear HMGB1 must leave the cell to enter the extracellular space where it can interact with various receptors to promote inflammation. Of these receptors, the RAGE was the first identified surface receptor for HMGB1. RAGE is expressed on both immune and nonimmune cells. Ligation of HMGB1 to RAGE activates diverse arrays of intracellular pathway cascades promoting cellular migration. In addition to RAGE, TLR-2 and TLR-4 have been recognized as surface receptor for HMGB1. TLR-2 and TLR-4 are expressed by monoytes, epithelial cells, and renal cells. HMGB1-mediated signaling through TLR-2 and TLR-4 leads to cytokine production. Recently, another TLR member, TLR-9, has been recognized as an intracellular receptor for HMGB1. TLR-9 is expressed by immune cells such as monocytes, B-lymphocytes, and mainly by plasmacytoid dendritic cells (pDCs). TLR-9 recognizes exogenous and endogenous DNA. HMGB1 complexed with DNA enhances cytokine production, mainly IFN-α, in pDCs and induces antibody production from B cells through interacting with TLR-9.2,3
HMGB1 in Diseases with Renal Manifestations
Exciting data indicate that HMGB1 can play an important role in the pathogenesis of chronic inflammatory and autoimmune diseases. Thus, there is evidence that the expression of HMGB1 is increased in the sera of patients in a wide variety of immunologically mediated conditions as well as at sites of local inflammation. In particular, the role of HMGB1 in renal diseases has been explored intensively. In an animal model of adenine-induced nephropathy, granuloma formation was found to be associated with high HMGB1 expression and up-regulation of RAGE and TLR-4 in the kidney. In addition, the presence of HMGB1 worsened the kidney function in rats with granulomatous nephritis.11
A potential influence of HMGB1 on kidney function has also been demonstrated in humans. In patients with chronic kidney disease (CKD), levels of HMGB1 were negatively correlated with glomerular filtration rate (GFR) suggesting that elimination of this protein may be at least partially mediated via the kidney. In this study, serum HMGB1 levels were correlated with inflammatory markers (TNF-α, hs-CRP, IL-6), suggesting that HMGB1 might be used as a predictor of CKD outcome.12 Furthermore, HMGB1 was shown to be expressed in the sera of patients with vasculitis, IgA nephritis, and glomeruloneprhritis (GN) associated with antineutrophilic cytoplasmic antibody (ANCA).13
The significance of HMGB1 in ANCA-associated vasculitic disorders (granulomatosis with polyangiitis [Wegener’s], microscopic polyangiitis [MPA],and Churg-Strauss syndrome [CSS]) has also been investigated.13,15 ANCA-associated disorders are inflammatory conditions of small–medium -sized vessels known to affect kidneys. These conditions are characterized by focal necrotizing and/or crescentic pauci-immune glomerulonephritis. Since HMGB1 is released from necrotic cells, its role as a potential mediator of inflammation and damage has been investigated in these disorders. As results of these studies showed, serum HMGB1 levels are significantly increased in patients with active granulomatosis with polyangiitis but not in active MPA; these findings suggest that determination of serum HMGB1 levels could be important clinically in differentiating between active forms of ANCA-associated vasculitis.15
In contrast to this finding, Bruchfeld and colleagues found that serum HMGB1 levels were increased in all patients with active ANCA-associated vasculitis; however, this increase was not specific to certain form of vasculitis and there was no difference in serum HMGB1 levels between subgroups.13 This difference of findings of the studies could be explained by the method used for detection of serum HMGB1 (i.e., ELISA vs. Western blot) and differences in disease characteristics.
Lupus Nephritis
Systemic lupus erythematosus (SLE) is a classical immune-complex disorder characterized by type III mechanism in association with the production of a wide array of autoantibodies. Patients with SLE present with many different manifestations that vary in intensity and severity. Among these manifestations, nephritis is among the most serious and dangerous clinical problems, often progressing to end-stage disease with associated morbidity and mortality.
While the basis of lupus nephritis has been extensively studied, the underlying pathogenetic mechanisms remain elusive and there is still uncertainty concerning the key events that lead to glomerular inflammation and damage. Importantly, immune complexes have long been considered key effectors in the induction of renal damage in lupus patients. Data for this mechanism derive from several key observations. Thus, studies using immunofluorescence microscopy have revealed immunoglobulin disposition at sites of injury in the lupus kidney. Among these antibodies, antibodies against DNA are the hallmark of the disease and have been shown to correlate with the SLE Disease Activity Index (SLEDAI). These antibodies can participate in the formation of immune complexes that can be deposited in tissues such as the skin and kidney; this deposition, in turn, activates complement to initiate inflammatory responses.2,16
A variety of mechanisms could explain the role of anti-dsDNA antibodies in promoting renal disease and their activity as nephrogenic or nephritogenic effector. Thus, these antibodies have the ability to bind to native glomerular structures as well as to endothelial cells. Once these immune complexes deposit in the kidney or penetrate into cells, they can act in the following ways:
- Modulate cell viability and induce apoptosis;
- Affect the proinflammatory pathways, up-regulating adhesion factors (ICAM, VCAM) in endothelial cells and IL-1, IL-8 mRNA expression in mononculear cells, and enhance IL-6 expression in glomerular mesangial cell; and
- Activate complement system via classical pathway leading to further tissue injury.
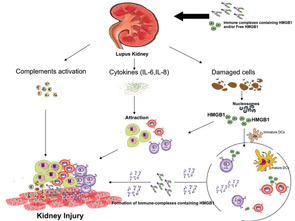
These effects are exerted directly by anti-dsDNA antibody via binding to Fc-receptor, which is present on the surface of many types of cell. In this situation, the receptor binds to the Fc region of antibody directly, or indirectly by DNA-containing immune complexes (via DNA antigen).16 Recently, it has been shown that these DNA-containing immune complexes also contain HMGB1, which is important for binding of these immune complexes to renal tissue and initiating renal injury in SLE patients.17
The potential role of HMGB1 in the pathogenesis of SLE is increased based on the fact that apoptotic cells accumulate in SLE and are the main source of autoantigens (nucleosomes) that may have bound HMGB1. Nucleosomes isolated form SLE sera were shown to be associated with HMGB1. Moreover, it has been shown that serum HMGB1 levels are increased in Chinese SLE patients compared with healthy controls and are correlated with disease activity and clinical parameters such as complements C3 and C4.18,19 In these studies, the investigators demonstrated that the elevation of HMGB1 levels was not associated with specific organ involvement. Also, Sato et al showed that sera of lupus nephritis patients didn’t show the tendency to be positive for HMGB1.13 However, in an IFN-accelerated murine model of SLE, administration of an anti-HMGB1 monoclonal antibody showed inhibition of proteinuria and led to kidney protection. These findings suggest that HMGB1 may play a role in the renal pathology. In contrast to previous studies on human SLE, our preliminary data show increased serum HMGB1 levels in active lupus nephritis patients.20 In addition, our data reveal a correlation of serum HMGB1 levels with proteinuria. The differences in the results of the various studies could reflect the 1) number and characteristics of patients included in the study; and 2) methods used to detect circulating HMGB1 (i.e., ELISA and Western blot).
Further supporting the involvement of HMGB1 in renal pathology are immunohistochemical studies performed on kidney tissue from lupus nephritis patients. These studies have revealed increased HMGB1 expression. It was shown that HMGB1 expression was greater in lupus kidneys compared to controls and it was mainly observed in glomerular cells, tubular cells, as well as infiltrates of mononuclear cells.17 Despite these findings, the questions regarding the origin of HMGB1 in kidney tissues as well as its precise role in pathogenesis remain unresolved. Thus, it is possible that circulating HMGB1 in its free form or complexed form (i.e., DNA-containing immune complexes) binds to RAGE and TLRs (2, 4, and 9) expressed on renal tissue. This binding could initiate an inflammatory response. In this case, the HMGB1 could be produced outside the kidney and arrive via the immune complexes, almost as a Trojan horse. Alternatively, damaged renal cells could secrete HMGB1, creating a local supply that has the ability to act as chemokine, attracting monocytes and lymphocytes. These cells could further secrete cytokines amplifying the inflammation (see Figure 2).
Conclusion
As inflammatory mediator and prototypic alarmin, HMGB1 could play an important role in the pathogenesis of autoimmune diseases especially those with renal manifestations. In SLE, immune complex–mediated nephritis is considered the main cause of mortality. Cellular and molecular mechanisms underlying the pathogenesis of lupus nephritis are still unknown. Considering the data discussed here, HMGB1, whether in its free or complexed form (as components of immune complexes), could play an important role in the pathogenesis of lupus nephritis. It is tempting to speculate that measurement of HMGB1 (in the blood or possibly the urine) could represent a potentially useful new marker for kidney disease that could signify both inflammation and damage.
In addition to highlighting the value of HMGB1 as a biomarker, ongoing studies are investigating this protein as a new therapeutic target in SLE. This role is consistent with studies on the efficacy of strategies to reduce HMGB1 levels in experimental arthritis and shock in animals. While much work on HMGB1 remains for the future, the study of this dual function protein has nevertheless provided important new insights into the operation of the innate immune system and the way in which nuclear molecules in lupus can promote inflammation.
Clearly, in the immune system as in real estate, location is everything and, when HMGB1 escapes from the confines of the nucleus, it takes on another identity and can drive inflammation in a way entirely unexpected for a protein of this kind. The future will tell whether alarmins can be targets of new types of immunosuppression, demonstrating a safety and toxicity profile that can be a meaningful advance over that of current agents. the rheumatologist
Drs. Abdulahad and Bijl are faculty in the department of rheumatology and clinical immunology at the University Medical Center Groningen at the University of Groningen in The Netherlands.
References
- Harris HE, Raucci A. Alarmin(g) news about danger: Workshop on innate danger signals and HMGB1. EMBO Rep. 2006;7:774-778.
- Abdulahad DA, Westra J, Limburg PC, Kallenberg CG, Bijl M. HMGB1 in systemic lupus erythematosus: Its role in cutaneous lesions development. Autoimmun Rev. 2010;9:661-665.
- Pisetsky DS, Erlandsson-Harris H, Andersson U. High-mobility group box protein 1 (HMGB1): An alarmin mediating the pathogenesis of rheumatic disease. Arthritis Res Ther. 2008;10:209.
- Bell CW, Jiang W, Reich CF, III, Pisetsky DS. The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol. 2006;291:C1318-C1325.
- Wang H, Vishnubhakat JM, Bloom O, et al. Proinflammatory cytokines (tumor necrosis factor and interleukin 1) stimulate release of high mobility group protein-1 by pituicytes. Surgery. 1999;126:389-392.
- Andersson U, Wang H, Palmblad K, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565-570.
- Dumitriu IE, Baruah P, Bianchi ME, Manfredi AA, Rovere-Querini P. Requirement of HMGB1 and RAGE for the maturation of human plasmacytoid dendritic cells. Eur J Immunol. 2005;35:2184-2190.
- Liu QY, Yao YM, Yan YH, Dong N, Sheng ZY. High mobility group box 1 protein suppresses T cell-mediated immunity via CD11c(low)CD45RB(high) dendritic cell differentiation. Cytokine. 2011;54:205-211.
- Huang LF, Yao YM, Zhang LT, Dong N, Yu Y, Sheng ZY. The effect of high-mobility group box 1 protein on activity of regulatory T cells after thermal injury in rats. Shock. 2009;31:322-329.
- Avalos AM, Kiefer K, Tian J, et al. RAGE-independent autoreactive B cell activation in response to chromatin and HMGB1/DNA immune complexes. Autoimmunity. 2010;43:103-110.
- Oyama Y, Hashiguchi T, Taniguchi N, et al. High-mobility group box-1 protein promotes granulomatous nephritis in adenine-induced nephropathy. Lab Invest. 2010;90:853-866.
- Bruchfeld A, Qureshi AR, Lindholm B, et al. High Mobility Group Box Protein-1 correlates with renal function in chronic kidney disease (CKD). Mol Med. 2008;14:109-115.
- Sato F, Maruyama S, Hayashi H, et al. High mobility group box chromosomal protein 1 in patients with renal diseases. Nephron Clin Pract. 2008;108:c194-c201.
- Bruchfeld A, Wendt M, Bratt J, et al. High-mobility group box-1 protein (HMGB1) is increased in antineutrophilic cytoplasmatic antibody (ANCA)-associated vasculitis with renal manifestations. Mol Med. 2011;17:29-35.
- Wibisono D, Csernok E, Lamprecht P, Holle JU, Gross WL, Moosig F. Serum HMGB1 levels are increased in active Wegener’s granulomatosis and differentiate between active forms of ANCA-associated vasculitis. Ann Rheum Dis. 2010;69:1888-1889.
- Mortensen ES, Rekvig OP. Nephritogenic potential of anti-DNA antibodies against necrotic nucleosomes. J Am Soc Nephrol. 2009;20:696-704.
- Qing X, Pitashny M, Thomas DB, Barrat FJ, Hogarth MP, Putterman C. Pathogenic anti-DNA antibodies modulate gene expression in mesangial cells: Involvement of HMGB1 in anti-DNA antibody-induced renal injury. Immunol Lett. 2008;121:61-73.
- Li J, Xie H, Wen T, Liu H, Zhu W, Chen X. Expression of high mobility group box chromosomal protein 1 and its modulating effects on downstream cytokines in systemic lupus erythematosus. J Rheumatol. 2010;37:766-775.
- Ma CY, Jiao YL, Zhang J, et al. Elevated plasma level of HMGB1 is associated with disease activity and combined alterations with IFN-alpha and TNF-alpha in systemic lupus erythematosus. Rheumatol Int. 2010. Dec 1. [Epub ahead of print]
- Abdulahad DA, Westra J, Bijzet J, Limburg PC, Kallenberg CG, Bijl M. High mobility group box 1 (HMGB1) and anti-HMGB1 antibodies and their relation to disease characteristics in systemic lupus erythematosus. Arthritis Res Ther. 2011;13:R71. [Epub ahead of print]