Storage of energetic fuels is mainly supported by anabolic pathways such as the parasympathetic nervous system with the vagus nerve (uptake in the gut and liver), insulin from the pancreas (uptake of glucose into liver, muscle, and fat tissue and storage of triglycerides), insulin-like growth factor–1 (for growth support), estrogens (support of fat tissue and growth), androgens (support of muscle tissue and growth), vitamin D and parathyroid hormone (support of bone and calcium and phosphate storage), and osteocalcin (support of insulin actions).1 Figure 1 (p. 25) summarizes neuroendocrine regulation of storage of energetic substrates.
Provision of energetic fuels is mainly mediated by catabolic pathways such as those regulated by the sympathetic nervous system (SNS; via β2-adrenoceptors, gluconeogenesis, and lipolysis); the hypothalamic-pituitary-adrenal (HPA) axis (cortisol, gluconeogenesis, and protein breakdown in muscles); and the hypothalamic-pituitary-thyroid axis (thyroid hormones support effects of the SNS) (see Figure 1, p. 25).
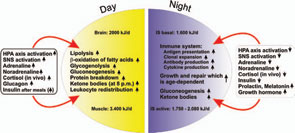
If all bodily organs consumed limiting resources simultaneously, a serious shortage would rapidly ensue, and insufficient energy would be available to sustain organ function. Regulation of energy allocation to stores or consumers is a delicate process. Indeed, the brain and muscles mainly need energetic fuels when active during the day, while immune system activity and growth processes are augmented during night. Neuroendocrine regulation of energy allocation follows a circadian rhythm (see Figure 2, p. 25).1 An important question thus arises: Have complex neuroendocrine immune interactions been positively selected during evolution in the context of CIDs, or do they have another basis?
Evolutionary Medicine
In the pathogenesis of symptomatic CIDs, genes that might play a specific role (favorable or unfavorable) may not have escaped conservation evolutionarily because unrestricted gene transmission was not possible for the following reasons:9
- High negative selection pressure. Thus, CIDs may lead to loss of reproducibility because affected individuals are at a disadvantage, excluded, or impaired in the competition for food, positions in the group, and sexual partners. In addition, long-term high-inflammatory activity may inhibit the hypothalamic-pituitary-gonadal axis, leading to impairment of fertility.10-14
- No selection pressure at all. At present, many CIDs only become manifest in patients at older ages. Due to the short life expectancy in the past, our ancestors may not have suffered from the CIDs that we know today.
- There was no time for natural selection. This situation would occur if various CIDs did not exist 100 to 200 years ago. Indeed, there is a suggestion that RA, for example, is a relatively new disease.
Nevertheless, genes can be transmitted before the outbreak of a CID and persist among descendants. On the other hand, genes whose products operate in the symptomatic phase of CIDs will not be positively selected due to negative influence of the disease (negative selection pressure). Transmitted genes may confer an increased risk for a CID, but, most likely, these genes were positively selected for fitness in reproduction and survival at younger ages independent of the CID (antagonistic pleiotropy).15 For example, HLA DR4 (DRB1*04), known to be a risk factor in RA, shows a high negative association with the risk of dengue hemorrhagic fever.16 It is possible, therefore, that HLA-DR4 (DRB1*04) was positively selected to fight off dengue hemorrhagic fever (or some other infection), although later in life, this particular surface molecule supports the development of a CID by increasing antigen presentation and T cell activation.9,17
It make sense that specific genes have not been selected or conserved in evolution because of their putative role in CIDs, either positive or negative. Rather, genes, signaling pathways, and networks operative in symptomatic CIDs were likely borrowed from serious, albeit non-life-threatening, inflammatory episodes, such as transient events. Recovery is possible from these episodes, but they would not have blocked the gene transmission to the progeny. According to these ideas, genes and the response programs they regulate are conserved for host defense against infection, control of inner and outer body surfaces, foreign body reactions, wound healing and repair, immunosurveillance in tissue, implantation of the stem cells into injured tissue, immune phenomena facilitating semiallogenic pregnancy, replacement of cells and tissue (physiological regeneration and degeneration), and similar ones. The same is true for neuroendocrine regulation of energy storage and energy allocation to consumers.
Energy Regulation and Evolutionary Medicine
In an acute inflammatory infectious disease, the immune system has a pressing need to boost its energy supply (see Table 1, at right). To meet this demand, neuroendocrine systems are switched on to provide energy-rich substrates to the activated immune system (the red hormone axes in Figure 1, p. 25). Most likely, these responses have been evolutionarily conserved for acute inflammatory episodes.
