During starvation (think of the transient inflammatory episode that was the basis of evolutionary conservation), metabolic support for survival involves first glycogen stores in the liver (for half a day), then protein stores in the muscles (one to three days), and finally fat stores in adipose tissue (from day three on). By utilizing all reserves, a patient of normal weight can live up to 2.5 months. In the starvation program, liver glycogen and muscle protein are first used. If the full starvation program is not started after three days, fat reserves will be spared, but proteins from muscles will be mainly utilized (recall that free fatty acids can not be used in gluconeogenesis). Since cachectic obesity with muscle loss and maintenance of adipose tissue is a hallmark of many CIDs, such an incomplete starvation program is most probably active in CIDs.21
Insulin Resistance with Hyperinsulinemia
Resistance to insulin affects the liver (reduced glucose uptake), muscle (reduced glucose uptake), and fat tissue (reduced glucose uptake and reduced triglyceride storage). However, the activated immune system does not become insulin resistant. In contrast, insulin upregulates glucose transporters on immune cells that support glucose uptake. Insulin resistance is an adaptive program for short-lived inflammatory episodes to redirect glucose and free fatty acids to activated immune cells and to stimulate immune responses.1 In other words, the energy highways to liver, muscle, and fat tissue are detoured to the activated immune system.
Dyslipidemia
Although the pattern of dyslipidemia may vary between different CIDs, a common phenomenon in all CIDs is a low level of high-density lipoprotein (HDL) cholesterol and/or apolipoprotein A-I.1 HDL is instrumental in removing cholesterol from tissue (reverse cholesterol transport). Loss of typical HDL cholesterol and appearance of a proinflammatory form of HDL with decreased apolipoprotein A-I and increased serum amyloid A and ceruloplasmin is augmented in CIDs.22 In the context of acute inflammatory episodes, the CID-related transition of normal HDL to proinflammatory HDL becomes understandable as part of an adaptive program.23 For energy regulation, this response is appropriate because it inverts reverse cholesterol transport and, thus, increases provision of energy-rich fuels to immune cells (think of the fat-laden macrophage).
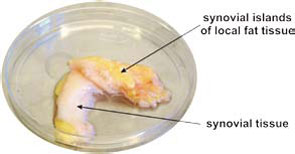
Increase of Adipose Tissue in the Proximity of Inflammatory Lesions
Many CIDs demonstrate fat deposits near inflammatory lesions such as creeping fat in Crohn’s disease or fat tissue adjacent to synovial tissue in RA (see Figure 3, p. 26). Because fibroblasts can easily transform into adipocytes, the appearance of fat tissue in the proximity of inflammatory lesions may be an adaptive program.24 Although several factors could lead to this type of transformation, locally increased estrogen levels could be an important mediator of this process.25 These fat deposits can nourish nearby inflammatory processes because immune cells directly use free fatty acids. This possibility is supported by an increased density of sympathetic nerve fibers in the juxtasynovial fat tissue of RA patients due to β2‑adrenergically induced lipolysis.26
Alterations of Steroid Hormone Axes
The well-known mild increase of the HPA axis activity in CIDs (albeit inadequately low to suppress chronic inflammation) with elevated cortisol levels is an adaptive program to support gluconeogenesis in the liver. In addition, this program redirects energy-rich fuels to the activated immune system by catabolic influences on muscle (protein degradation, amino acids for gluconeogenesis).
