Despite the innovations of new biologics and disease-modifying anti-rheumatic drugs, a large unmet need remains for patients with rheumatic autoimmune disease. Treatment remains limited for many conditions, including for conditions with a dim prognosis, such as systemic sclerosis.1 One promising treatment avenue is hematopoietic stem-cell transplantation (HSCT). Here, we provide background on HSCT for severe rheumatic autoimmune disease, examine the perspectives of three patients who sought out clinical trials and provide a research update on systemic sclerosis.
HSCT History & Current Status
In 1996, a patient with connective tissue disease and severe pulmonary hypertension underwent autologous HSCT—the first patient with rheumatic autoimmune disease reported to receive the treatment.2 Since that time, clinical research teams have performed HSCT for a number of different rheumatic autoimmune conditions, including systemic sclerosis, lupus, rheumatoid arthritis, juvenile idiopathic arthritis and, less commonly, for other conditions, such as Sjögren’s syndrome, mixed connective tissue disease, Behçet’s disease, psoriatic arthritis, ankylosing spondylitis and vasculitides.3,4
Worldwide, teams have performed over 3,000 HSCT procedures for rheumatic and nonrheumatic autoimmune disease. These have been mostly autologous HSCT, as documented through the Center for International Blood and Marrow Transplant Research and the European Group for Blood and Marrow Transplantation.3
Researchers adopted the technique of HSCT from the fields of hematology and oncology.4 HSCT aims to reset an autoreactive immune system. Current immunosuppressive and biological treatments for autoimmune rheumatological disease can successfully suppress immune response, but they are unable to target autoreactive immunologic memory. Moreover, they must be taken continuously in order to maintain immunosuppression. In contrast, HSCT therapies can potentially provide sustained, total remission of autoimmune syndromes without the need for ongoing therapy.

Dr. Georges Georges
HSCT profoundly depletes the autoreactive immunologic memory (T cells and B cells) via high-dose chemotherapy. Conditioning regimens vary. In general though, stem cells are mobilized into the bloodstream by such treatments as granulocyte colony-stimulating factor and later collected. Several weeks later, patients undergo some version of immunoablative therapy, receiving such agents as anti-thymocyte globulin and cyclophosphamide. During this time, the autoreactive immunological memory is profoundly depleted. This is followed by autologous stem cell transplant, which reconstitutes a new immune system and reestablishes immune tolerance.3
After transplant, T cells appear to be largely derived from the stem-cell graft, and T regulatory cells display a renewed repertoire of T cell receptors.3,5 Autoantibodies are also substantively reduced after HSCT treatment.5

Kathy Peel had HSCT for her lupus in July 2016.
Currently, multiple sclerosis, Crohn’s disease and systemic sclerosis are the autoimmune diseases with the most supporting clinical data, including information from large series and randomized controlled trials. Retrospective analyses support its possible efficacy for additional conditions.6 Currently, no official U.S. guidelines exist for HSCT for rheumatological disease treatment, but the European Society for Bone and Marrow Transplantation recommends autologous HSCT as part of clinical trials for the treatment of certain subgroups of patients with severe systemic sclerosis, systemic lupus and juvenile idiopathic arthritis.7
Ongoing Treatment Concerns
Although the therapy has a great deal of potential, many clinicians are concerned about the risk/benefit ratio. The conditioning regimen puts patients at risk of complications, such as infection, secondary autoimmune disease and malignancy.8 Some studies have shown an increase in short-term mortality following HSCT, but safety of HSCT has improved since its beginnings.8,9 Researchers continue to work to develop regimens that are effective, but less toxic.
Georges Georges, MD, is an associate member at Fred Hutchinson Cancer Research Center and an associate professor of medicine at the University of Washington, both in Seattle. He is part of a team that has performed such transplants in patients with severe autoimmune disease.
“There is always a competition between transplant and nontransplant therapy to find the most effective therapy that causes the least risk and discomfort. There is always the hope that there is some simpler drug therapy that can be developed [to] target specifically the cells responsible for the disease process.” He points out that this may be partly why research interest in HSCT has lessened for certain diseases that have more effective drug treatments, such as rheumatoid arthritis.
Patient Perspectives
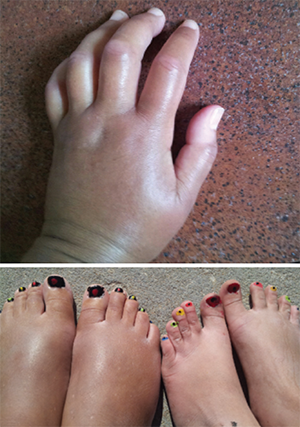
Ms. Gould was diagnosed with systemic sclerosis in 2015. These photos show her hands and feet (left) prior to the transplant.
What follows are perspectives from three women who received HSCT for disabling autoimmune conditions. All three received their treatment through Northwestern Feinberg School of Medicine’s Division of Immunotherapy for autoimmune disease.
HSCT & Lupus: Kathy Peel is a 36-year-old woman, a sales analyst, wife and mother of two. She was diagnosed with lupus in 2005.
Ms. Peel’s doctors had an extremely difficult time getting her disease under control, even with treatment. Her lung function had significantly deteriorated. Her doctor told her that she would never be able to come off prednisone and her disease would probably never go into remission.
Ms. Peel and her mother began researching alternatives. Ms. Peel says, “I found a YouTube video of a girl who had basically the same story and was in remission after HSCT. I connected with a group of people on Facebook who had either had the procedure or wanted to have it done, and I found out most of my information through them.”
As part of her evaluation for the procedure, she discovered that she had a problem with her heart valve related to her lupus, which motivated her even more to explore HSCT. She was eventually accepted as part of the HSCT trial, but she couldn’t get her insurance to approve it. “We [had] to pay out of pocket. They strung it out for months. This whole time I was thinking, ‘What if my heart is getting worse?’ So my mom and dad said, ‘We are going to do whatever we have to do to get the money. We aren’t waiting on insurance.’”
Experts generally agree that autologous HSCT is often able to induce sustained clinical remission in people with lupus. However, many of the currently available studies of HSCT in patients with lupus have shown an increase in short-term mortality.10
Ms. Peel went through the procedure in July 2016. Since the procedure, she has been able to stop taking all of her medications for lupus. Her lung function and overall health have improved. “What I went through in those three months has given me a year of my life at this point that I didn’t have before,” she explains. “I’ve missed a lot with my kids. My relationship suffered. It takes a toll on everybody around you, not just you as a person.”
Ms. Peel says her local doctor did not support her exploring HSCT as a possibility. She adds that doctors need to consider quality-of-life issues as they talk through possible treatment options with their patients. “They need to understand that just because the person is living that that is not true living. If they are to a point like I was—there was nothing left for me to do but this.”
Although [hematopoietic stem-cell transplantation] has a great deal of potential, many clinicians are concerned about the risk/benefit ratio of the procedure. The conditioning regimen puts patients at risk of complications, including infection, secondary autoimmune disease & malignancy.
HSCT & Stiff Person Syndrome: Whitney Blake is a 32-year-old journalist. For almost 10 years, she has experienced symptoms of stiff person syndrome (SPS). This condition is incredibly rare, occurring in perhaps one or two people per million, although it may be underdiagnosed.11,12
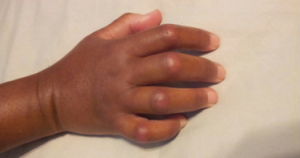
Ms. Gould underwent HSCT in summer 2016, after this photo was taken.
SPS causes increasingly severe muscle stiffness, rigidity and spasms of the axial muscles. Ultimately, it can cause significant disability and occasionally acute respiratory failure.13 The syndrome is thought to have an autoimmune basis, with the large majority of patients having glutamic acid decarboxylase (GAD) autoantibodies. Benzodiazepines and other GABA-acting medications can be used to help with stiffness and spasms, along with muscle relaxants and immune-modulating therapies like IVIG and plasma exchange.11,12
Among other problems, Ms. Blake experienced nerve pain, vision issues and abdominal muscle spasms. She saw several neurologists before a rheumatologist finally diagnosed her with SPS—seven years after she sought medical care, the average time to diagnosis in SPS patients. Although neurologists are often the primary caregivers for people with SPS, rheumatologists can also aid in diagnosis and treatment. Ms. Blake notes that, in her experience, rheumatologists are “more out-of-the-box thinkers” and so may be of particular help with diagnosing rare conditions such as SPS.

Whitney Blake underwent HSCT in summer 2016 to treat her stiff person syndrome.
“I try to educate nurses, med students, interns, residents and doctors about the symptoms in hopes that they will recognize it in another patient, and perhaps we’ll get that average time of diagnosis down to a shorter period of time,” Ms. Blake explains. “That is important, because the longer you have the disease without being treated, and the longer it progresses, the worse off you will be.”
Even with proper diagnosis and treatment, Ms. Blake’s symptoms were progressing. As part of her research, Ms. Blake discovered the SPS clinical trial at Northwestern. “I was going downhill, and the doctors didn’t really have anything else except high-dose Cytoxan [cyclophosphamide]. So my doctors and I thought, we might as well get the stem cell transplant done.”
It’s difficult to research rare diseases, such as SPS. Outcomes for HSCT and SPS are still extremely limited. A 2014 case report from the Ottawa Blood and Marrow Transplant Program showed remission of symptoms in two patients with severe SPS who received autologous HSCT, using the same regimen used to treat patients with multiple sclerosis (MS).14
After an initial evaluation, Ms. Blake was accepted into the trial and went through the procedure in the summer of 2016. She considers herself fortunate that she did not have to make multiple appeals to her insurance company. “I did pretty well,” she says, crediting the excellent care that she received from her medical team at Northwestern. “It wasn’t as bad as I thought it would be.” She remains hopeful about the potential long-term benefits.
Ms. Blake thinks many doctors less familiar with HSCT are thinking about the risks of allogeneic transplants, not autologous transplants. “Here, you are using your own stem cells. This is much safer. Some neurologists are coming around to seeing this as a very good treatment for some MS patients, for example.”
For a rare, disabling disease like SPS, HSCT may provide an important option for patients, moving forward. “A lot of people hear about it from friends instead of their doctors,” adds Ms. Blake. “It is definitely a good thing for doctors to have HSCT on their radar as a potential therapy.”
HSCT & Systemic Sclerosis: Julie Gould is a 46-year-old woman, a nurse and a mother of three. After several years of misdiagnosis, she was eventually diagnosed with systemic sclerosis in 2015. She experienced extreme fatigue and swelling of her hands and feet. Her lung function was beginning to deteriorate, and she also was diagnosed with gastric antral vascular ectasia (GAVE).
“All of the information that I had seen on the Internet and that my local rheumatologist had given me was pretty dismal. It pretty much told me that if I took good care of myself and watched my blood pressure that I would be lucky if I lived five years,” Ms. Gould notes. “My mom and I were obsessed about finding information about this disease. We just happened to come across an article on stem cell transplant, and we started researching it.”
Eventually, she learned about the systemic sclerosis trial at Northwestern. During an open enrollment period, she switched to an insurance plan that would cover a clinical trial for stem cell treatment. She underwent HSCT in summer 2016.
Ms. Gould had the transplant before her lung function had deteriorated significantly. Because of her borderline symptoms, her medical team offered her the option of waiting to have the transplant. Ms. Gould opted to proceed. She explains, “I wanted to ahead and have it because I didn’t want to get any worse. Because the transplant only stops the progression—it doesn’t necessarily improve any of the conditions that you have that have already gotten bad.”
Ms. Gould notes, “I didn’t have a lot of the issues that some of the people had. I pretty much went through it without a lot of complications.” She believes part of that is because she had the procedure before her symptoms became too severe.
Ms. Gould was pleased with the outcome of her HSCT, and she recommends it to others in a similar situation. Her pulmonary function tests have stabilized, and she notes, “My feet are the size that they were before I got sick. I do feel so much better.”
Ms. Gould believes it is important for doctors to take a balanced approach when discussing HSCT with their patients. “I have taken care of oncology patients in the past as a nurse, so I knew how sick those patients can be. So I can see why they would not be an advocate for stem cell transplant from that point of view.” However, Ms. Gould believes it is important for doctors not to discourage patients about the procedure prematurely. Ms. Gould sought treatment even after both of her local doctors discouraged her from pursuing HSCT because they saw it as an experimental procedure. “I was willing to try something somewhat experimental,” she says.
SSc HSCT Research Update
To date, systemic sclerosis is the rheumatic autoimmune disease with the most empirical support. Many studies have provided evidence that HSCT can potentially provide effective treatment for systemic sclerosis.15-20 The ASTIS trial was a Phase 3 randomized trial of 156 patients with diffuse cutaneous systemic sclerosis. HSCT in these patients gave a significant long-term survival benefit, although it was associated with increased treatment-related mortality in the first year after treatment.21

Julie Gould
More recently, researchers presented the results of the SCOT (cyclophosphamide or transplantation) trial at the 2016 ACR/ARHP Annual Meeting. The results are currently pending publication. Keith Sullivan, MD, is principal investigator of the trial, which studied 75 patients randomized to either cyclophosphamide (750 mg/m2/mo) or myeloablation (800 cGy total body irradiation with lung and kidney shielding, 120 mg/kg cyclophosphamide and 90 mg/kg anti-thymocyte globulin), followed by CD34+selected autologous HSCT. The trial demonstrated superiority of the HSCT treatment on clinical outcomes, with patients receiving HSCT showing a significant reduction in the use of disease-modifying anti-rheumatic drugs.
Dr. Georges, one of the study collaborators, explains, “We now have three randomized clinical trials that demonstrate the superiority of transplant over conventional chemotherapy, with the transplant arm having a superior overall survival. This is convincing evidence that autologous hematopoietic stem cell transplant is effective therapy for systemic sclerosis.” He continues, “People at both meetings I attended said this was a practice-changing study, that this should really give courage to patients who have significant diffuse systemic sclerosis with declining pulmonary function and increasing skin scores despite other treatments.”
Experts generally agree that autologous HSCT is often able to induce sustained clinical remissions in people with lupus.
In terms of the risk/benefit ratio of HSCT for systemic sclerosis, Dr. Georges is encouraged by the fact that in the SCOT trial there were no transplant-related deaths within the first 100 days after transplant. (There were, however, two patients who later died from myelodysplastic syndrome, one at 15 months post-transplant and another 5.7 years post-transplant.) In contrast, 10% of patients in the HSCT arm of the ASTIS trial died from treatment complications within 100 days of transplant. “I think many rheumatologists were disappointed with this mortality outcome from ASTIS,” he notes.
Dr. Georges speculates the difference may have arisen from varying conditioning regimens between the studies. For the pretransplant regimen, the ASTIS trial used a higher dose of cyclophosphamide (200 mg/kg). In contrast, the SCOT trial used a lower dose (120 mg/kg). “What is interesting is that radiation plus cyclophosphamide at 120 mg/kg from the SCOT trial seemed to be better tolerated than the ASTIS regimen. The SCOT trial had fewer patients in the ICU, no patients with early transplant deaths and also an excellent disease response.”
Dr. Georges hopes treatment centers will begin making HSCT a standard treatment option for systemic sclerosis patients who meet specific criteria, not solely as part of clinical trials. This may also increase the number of patients receiving insurance approval for the procedure. He notes that the SCOT trial had trouble accruing patients because of lack of approval by insurance companies. “Over 200 patients tried to get into the study, but only about 75 patients ended up having insurance approval. That was frustrating for patients, physicians and researchers.”
Dr. Georges encourages rheumatologists to develop close educational relationships with their patients, helping steer them to reputable centers for these treatments. He also recommends referring physicians seek transplant evaluation before the disease is too advanced. “Patients with disease factors of poor prognosis should be referred to transplant ASAP, because the earlier you do it, the less organ damage there is,” says Dr. Georges. “And there is a lower risk of death from transplant. Once organ function is very impaired by the disease process, there is a greater risk of transplant mortality.”
Moving forward, as HSCT for systemic sclerosis moves into more standard treatment, other severe autoimmune diseases may follow. Dr. Georges adds, “There are many rare neurologic autoimmune diseases that weren’t well recognized even 20 years ago, and some of these appear to be responding very well to HSCT. There may be other less frequently observed rheumatological diseases that may benefit, especially if there is not an effective treatment available for them.”
Ruth Jessen Hickman, MD, is a graduate of the Indiana University School of Medicine. She is a freelance medical and science writer living in Bloomington, Ind.
References
- Chang C. Unmet needs in the treatment of autoimmunity: From aspirin to stem cells. Autoimmun Rev. 2014 Apr–May;13(4–5):331–346.
- Tamm M, Gratwohl A, Tichelli A, et al. Autologous haemopoietic stem cell transplantation in a patient with severe pulmonary hypertension complicating connective tissue disease. Ann Rheum Dis. 1996 Oct;55(6):779–780.
- Swart JF, Delemarre EM, van Wijk F, et al. Haematopoietic stem cell transplantation for autoimmune diseases. Nat Rev Rheumatol. 2017 Apr;13(4):244–256.
- Tyndall A, Saccardi R. Haematopoietic stem cell transplantation in the treatment of severe autoimmune disease: Results from phase I/II studies, prospective randomized trials and future directions. Clin Exp Immunol. 2005 Jul;141(1):1–9.
- Alexander T, Arnold R, Hiepe F, Radbruch A. Resetting the immune system with immunoablation and autologous haematopoietic stem cell transplantation in autoimmune diseases. Clin Exp Rheumatol. 2016 Jul–Aug;34(4 Suppl 98):53–57.
- Kelsey PJ, Oliveira MC, Badoglio M, et al. Haematopoietic stem cell transplantation in autoimmune diseases: From basic science to clinical practice. Curr Res Transl Med. 2016;64(2):71–82.
- Sureda A, Bader P, Cesaro S, et al. Indications for allo- and auto-SCT for haematological diseases, solid tumours and immune disorders: Current practice in Europe, 2015. Bone Marrow Transplant. 2015 Aug;50(8):1037–1056.
- Daikeler T, Tichelli A, Passweg J. Complications of autologous hematopoietic stem cell transplantation for patients with autoimmune diseases. Pediatr Res. 2012 Apr;71(4 Pt 2):439–444.
- Atkins HL, Muraro PA, van Laar JM, Pavletic SZ. Autologous hematopoietic stem cell transplantation for autoimmune disease—Is it now ready for prime time? Biol Blood Marrow Transplant. 2012 Jan;18(1 Suppl):S177–S183.
- Illei GG, Cervera R, Burt RK, et al. Current state and future directions of autologous hematopoietic stem cell transplantation in systemic lupus erythematosus. Ann Rheum Dis. 2011 Dec;70(12):2071–2074.
- Hadavi S, Noyce AJ, Leslie RD, Giovannoni G. Stiff person syndrome. Pract Neurol. 2011 Oct;11(5):272–282.
- Helfgott SM. Stiff-man syndrome: From the bedside to the bench. Arthritis Rheum. 1999 Jul;42(7):1312–1320.
- Jachiet V, Laine L, Gendre T, et al. Acute respiratory failure in a patient with stiff-person syndrome. Neurocrit Care. 2016 Dec;25(3):455–457.
- Sanders S, Bredeson C, Pringle CE, et al. Autologous stem cell transplantation for stiff person syndrome: Two cases from the Ottawa blood and marrow transplant program. JAMA Neurol. 2014 Oct;71(10):1296–1299.
- Binks M, Passweg JR, Furst D, et al. PhaseI/II trial of autologous stem cell transplantation in systemic sclerosis: Procedure related mortality and impact on skin disease. Ann Rheum Dis. 2001 Jun;60(6):577–584.
- Farge D, Passweg J, van Laar JM, et al. Autologous stem cell transplantation in the treatment of systemic sclerosis: Report from the EBMT/EULAR Registry. Ann Rheum Dis. 2004 Aug;63(8):974–981.
- Nash RA, McSweeney PA, Crofford LJ, et al. High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for severe systemic sclerosis. Blood. 2007 Aug 15;110(4):1388–1396.
- Vonk MC, Marjanovic Z, vanden Hoogen FH, et al. Long-term follow-up results after autologous haematopoietic stem cell transplantation for severe systemic sclerosis. Ann Rheum Dis. 2008 Jan;67(1):98–104.
- Burt RK, Shah SJ, Dill K, et al. Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): An open-label, randomised phase 2 trial. Lancet. 2011 Aug 6;378(9790):498–506.
- Burt RK, Oliveira MC, Shah SJ, et al. Cardiac involvement and treatment-related mortality after non-myeloablative haemopoietic stem-cell transplantation with unselected autologous peripheral blood for patients with systemic sclerosis: A retrospective analysis. Lancet. 2013 Mar 30;381(9872):1116–1124.
- Van Laar JM, Farge D, Sont JK, et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: A randomized clinical trial. JAMA. 2014;311(24):2490–2498.