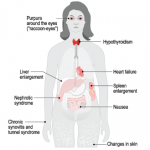
The authors identified only one clear adverse event in the human study: a moderate infusion-related reaction in the patient who received 0.5 mg/kg of ALN-TTR02. Mild-to-moderate infusion-related reactions occurred in 20.8% of patients receiving ALN-TTR01 and 7.7% of patients receiving ALN-TTR02.
Dr. Suhr expressed hope that it may be possible to bring the RNAi therapy to market in 2017 or 2018.
ad goes here:advert-1
ADVERTISEMENT
SCROLL TO CONTINUE
Dr. Pullen is a medical writer based in the Chicago area.
Reference