(Reuters)—The malaria treatment repeatedly championed by U.S. President Donald Trump as a game changer in the fight against the novel coronavirus has again failed to show a benefit in patients hospitalized with COVID-19, according to a recent study.
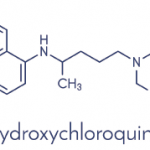
Although the study published in The New England Journal of Medicine had certain limitations, doctors report that the use of hydroxychloroquine (HCQ) lessened neither the need for mechanical ventilation nor the risk of death.
“We didn’t see any association between getting this medicine and the chance of dying or being intubated,” lead researcher Dr. Neil Schluger tells Reuters in a telephone interview. “The patients who got the drug didn’t seem to do any better.”
Among patients given HCQ, 32.3% ended up needing a ventilator or dying, compared with 14.9% of patients who were not given the drug.
But doctors were more likely to give HCQ to sicker patients, so researchers at New York-Presbyterian Hospital and Columbia University Irving Medical Center adjusted the rates to account for that. They concluded that the drug may not have hurt patients, but it clearly did not help.
Decades old HCQ, which is also used to treat lupus and rheumatoid arthritis, also showed no benefit when combined with the antibiotic azithromycin, Schluger’s team reported. Azithromycin alone also showed no benefit.
Last month, doctors at the U.S. Department of Veterans Affairs reported that HCQ did not help COVID-19 patients and might pose a higher risk of death.
That analysis of medical records showed a death rate of 28% when the drug was given in addition to standard treatments, compared to 11% with standard care alone.
In the latest study, 811 patients got HCQ and 565 did not.
Because they were not randomly assigned to receive HCQ or a placebo, “the study should not be taken to rule out either benefit or harm” for the drug, researchers said. Randomized trials, the gold standard for tests of new therapies, should continue, they added.
But for now, “the guidance in our hospital has changed so we don’t recommend giving HCQ to hospitalized patients,” says Dr. Schluger, chief of the division of pulmonary, allergy and critical care medicine at Irving.
Smaller studies, including one done in China, had suggested HCQ might be useful, “but these were tiny studies and not of good quality. People seized on them because patients were dying,” he says.
There are currently no approved treatments for COVID-19, although Gilead Sciences Inc’s experimental antiviral drug remdesivir last week receive emergency use authorization from U.S. regulators.