This may be difficult to distinguish from the cartilage interface sign, which is an artifact. Cartilage interface sign is a thin, markedly hyperechoic line at the interface between the normal hyaline cartilage and an overlying abnormally hypoechoic or anechoic material, which is caused by a marked difference in acoustic impedances of the two tissues (e.g., effusion and hyaline cartilage).6
The diagnosis of malignant effusion should be considered in patients with HTLV-1-associated ATLL who present with joint swelling.
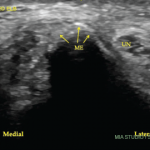
Our patient had ultrasound findings of a possible DC sign on anterior humero-radial longitudinal view. When this image alone was presented to a handful of ultrasound experts, five out of seven thought this represented a DC sign. The other two felt this was a cartilage interface sign. An OMERACT patient-based agreement and reliability exercise on lesions in gout found that intra- and interobserver reliability for DC was lower than for other elementary lesions of gout (tophus, erosions). The authors thought that one possible pitfall was the presence of cartilage interface sign in joints with effusion (for example in the knees).5
Dynamic imaging can help differentiate DC from cartilage interface sign. The cartilage interface sign should be present only when the insonation angle is 90º (when the ultrasound beam is perpendicular to the surface of the cartilage). The DC sign should also be present in areas where the insonation angle is <90º and should move with the cartilage during dynamic imaging because the crystals are deposited on the surface of the cartilage.
Another helpful maneuver is compression with the probe. If the effusion is compressed out of view, the cartilage interface sign should disappear, while the DC sign should remain.6
Finally, the presence of other elementary lesions of gout (erosion, tophus) increases the positive and negative predictive value for the correct diagnosis.7
One should also note that the DC sign has been described not only in patients with gout, but also in patients with asymptomatic hyperuricemia and that the presence of the DC sign correlates with the degree of hyperuricemia.8,9 However, to date no study has looked at synovial fluid from joints with DC sign from patients with asymptomatic hyperuricemia for the presence of uric acid crystals.
Conclusion
In summary, we present a rare case of suspected malignant synovitis in a patient six months after diagnosis of HTLV-1-associated ATLL. Most prior case reports describe malignant polyarthritis developing several years after initial ATLL diagnosis, rendering our patient’s accelerated clinical course potentially a unique presentation even within a rare condition. This may be confounded by a delay in workup in our patient; she only had one year of documented care in our system despite living locally for 12 years before then.