Currently, little is known about the sequence of events leading to new bone formation in patients with ankylosing spondylitis (AS). In rheumatoid arthritis, there is an association between inflammation and erosive bone destruction, which is not followed by repair.
For patients with AS, it has been proposed that a similar sequence of events takes place, with the modification that erosive bone destruction is followed by tissue repair, which develops into mature bone when inflammation has resolved.1 Testing this hypothesis is difficult because AS is a slowly progressing disease, and there is a lack of sensitive clinical and biochemical measures of new bone formation. Further, the anatomical location and complicated anatomy of the sacroiliac joints and spine limit the availability of biopsies for histopathological analyses.
Therefore, indirect methods, such as magnetic resonance imaging (MRI), have increasingly been used to investigate the relation between inflammation and new bone formation. This review provides a summary of the current knowledge on the relationship between inflammation and new bone formation assessed by MRI and radiography of the spine and sacroiliac joints (SIJs) of patients with AS.
MRI & Radiography
In contrast to conventional radiography, magnetic resonance imaging (MRI) can visualize inflammation in the bone marrow (i.e., bone marrow edema or osteitis), synovium, ligaments, entheses and soft tissue. Moreover, MRI can visualize other types of lesions, such as fat infiltration, erosion, sclerosis and ankylosis. However, when new bone formation (i.e., syndesmophytes and ankyloses) is assessed in the spine, conventional radiography still provides superior information to MRI because the longitudinal ligaments and the cortex of the vertebral bodies are all black and, therefore, difficult to differentiate from each other.2 Thus, MRI is the gold standard for assessment of inflammation and fat, and conventional radiography of the spine is the gold standard for assessment of new syndesmophytes and ankyloses.
MRI is the gold standard for assessment of inflammation & fat, & conventional radiography of the spine is the gold standard for assessment of new syndesmophytes & ankyloses.
On MRI, the T1-weighted sequence is superior for detection of fat, and it provides detailed information on the anatomy. Fat-suppressed sequences, such as short tau inversion recovery (STIR), are water sensitive and superior for detection of inflammation. MRI is based on a tomographic (i.e., slice-based) technique, which makes it possible to assess different types of lesions at different locations of the spine and SIJs, and compare these with findings in the same areas assessed with other imaging modalities.
Conventional radiography can only reliably detect new bone formation in the anterior part of the cervical and lumbar spine over at least two years and in the SIJs over four years.3,4 Abnormalities in the thoracic spine cannot be assessed reliably due to projections from overlapping structures from the large vessels, airways and ribs. Therefore, all studies with MRI and radiography are based on assessment of lesions located in the same anatomical areas, in the anterior part of the cervical and lumbar spine.
Spinal MRI lesions are defined by anatomical location, such as the vertebral corner, vertebral edge and vertebral unit, which differ in size. Vertebral corner lesions include only those lesions touching the vertebral corner. Vertebral edge lesions include lesions in the anterior half of the endplate, and vertebral unit lesions cover those lesions related to one or both endplates at a disk.
MRI lesions of inflammation and fat are defined according to standardized and well-established definitions based on signal intensity on different sequences.5 In the Canadian studies, erosion is defined as a breach in bone cortex with loss of bone matrix in the adjacent bone marrow, which is seen as a dark signal on T1-weighted MRI scans. In contrast, backfill is defined by a bright signal that appears as an eroded cavity (i.e., the dark signal has changed to a bright signal similar to the signal of fat tissue).6
Spinal MRI Inflammation & New Bone Formation
Four studies have investigated the association between spinal inflammation and new bone formation.7-10 In the first study, 39 AS patients initiating tumor necrosis factor-alpha (TNFα) inhibitor therapy were followed for two years.7 A higher proportion of vertebral edges with MRI inflammation at baseline developed new syndesmophytes after two years than did vertebral edges without MRI inflammation at baseline (6.5% vs. 2.1%, p=0.006). Further, vertebral edges with MRI inflammation were three times as likely to develop new syndesmophytes than vertebral edges without inflammation (odds ratio [OR] 3.3; 95% CI: 1.5–7.4). At baseline, there was no difference in the proportion of vertebral edges with and without inflammation that were associated with structural changes on conventional radiography (17.6% vs. 15.6%, p=0.5).
Another study investigated 41 AS patients treated with either TNFα inhibitor or standard therapy (i.e., nonsteroidal antiinflammatory drugs [NSAIDs] and/or physical therapy).8 The proportion of vertebral corners developing new syndesmophytes two years later was significantly higher in vertebral corners with MRI inflammation at baseline than in vertebral corners without inflammation at baseline (25% vs. 2.8%, p<0.0001). Additional subanalyses revealed that none of the vertebral corners with MRI inflammation at baseline and at follow-up two years later developed new syndesmophytes (0%) compared with vertebral corners with resolved inflammation (i.e., inflammation that disappeared from baseline) to follow-up (25%, p<0.0001) and vertebral corners without inflammation at baseline (4.9%, NS).
The third report tested the hypothesis that MRI inflammation in a vertebral corner that completely resolves is more likely to develop into new syndesmophytes than a vertebral corner without MRI inflammation.9 For patients treated with TNFα inhibitor therapy (n=23), new syndesmophytes developed more frequently from vertebral corners where MRI inflammation had completely resolved two years later (42.9%) than from corners where there was no inflammation at baseline or follow-up MRI (2.4%, p<0.0001). For patients treated with standard therapy (n=27), the results were not significant. Moreover, the study confirmed that vertebral corners with persistent inflammation don’t develop into new syndesmophytes (0%).
The last study included 177 patients from the ASSERT study, which was a 24-week, randomized, controlled trial comparing infliximab with placebo for the treatment of AS, with an open-label extension of two years.10 Vertebral units with inflammation at baseline were associated with development of new syndesmophytes after two years (OR 2.15). Vertebral units with persistent inflammation did not develop new syndesmophytes. On the patient level, there was no association between MRI inflammation score as assessed with the ankylosing spondylitis spinal MRI activity score (ASspiMRI-a) and structural damage progression as assessed with the modified Stoke AS Spine score (mSASSS) on radiography.
Despite the use of different methods, the results show that there is an association between spinal MRI inflammation at baseline and, in particular, between resolution of inflammation and development of new syndesmophytes in patients with AS. In contrast, persistent spinal inflammation is not associated with new bone formation.
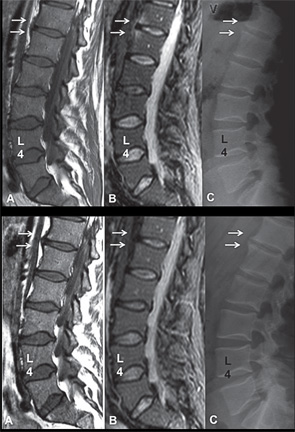
Spinal MRI Inflammation, Fat & New Bone Formation
The association between spinal MRI inflammation, fat and the development of new syndesmophytes has been assessed comprehensively in three studies.11-13 Based on the observation that MRI inflammation is often followed by fat infiltration in the spine and SIJs, the first study investigated whether the presence of fat infiltration in a vertebral corner is more likely to be associated with the development of a new syndesmophyte two years later than a vertebral corner without inflammation.12
The study included 100 AS patients treated with either a TNFα inhibitor or standard therapy. Using multivariate regression analysis, the study demonstrated that MRI inflammation independently predicted the development of new syndesmophytes (5.8 [2.2 to 15.3], p<0.001). MRI fat infiltration was also associated with new syndesmophyte formation in univariate (2.2 [1.1 to 4.5] p=0.03), but not in the multivariate (1.9 [0.9 to 4.1] p=0.1), analysis. Moreover, the presence of syndesmophytes/ankyloses on baseline radiography, a well-known independent predictor for new bone formation, was also significantly associated with the development of new syndesmophytes (multivariate analysis: 1.1 [1.1 to 1.3] p=0.01), although not as strongly as MRI inflammation.
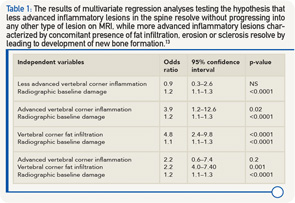
The same group of researchers also tested the hypothesis that “less advanced” inflammatory lesions in the spine resolve without progressing to any other type of lesion on MRI, while “more advanced” inflammatory lesions, characterized by concomitant presence of fat infiltration, erosion or sclerosis, resolve by leading to development of new bone formation.13 All models studied radiographic damage at baseline (i.e., syndesmophytes/ankyloses) as an independent variable and the development of new syndesmophytes two years later as a dependent variable. A total of 76 patients treated with a TNFα inhibitor were studied. In a model including less advanced vertebral corner inflammation, only radiographic damage independently predicted new bone formation (see Table 1). In a model with advanced vertebral corner inflammation, both inflammation and radiographic damage were independent predictors. In a model with vertebral corner fat lesions, both fat and radiographic damage were significantly associated with new bone formation. In a final model including both advanced corner inflammation and fat, only corner fat lesions and radiographic damage were independently associated with new bone formation.
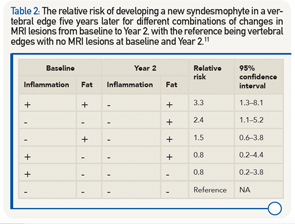
The relationship between changes in MRI inflammation, fat and development of new syndesmophytes was investigated in 73 AS patients from the ASSERT study.11 The relative risk for developing a new syndesmophyte in a vertebral edge five years later was calculated for different combinations of changes in MRI lesions from baseline to Year 2, with the reference being vertebral edges with no MRI lesions at baseline and Year 2. The highest relative risk was found for vertebral corners with both inflammation and fat at baseline and fat after two years (see Table 2). The relative risk was lower for combinations of lesions where fat was present at either baseline or after two years, and the lowest risk was found for vertebral corners with inflammation only at baseline.
Fat lesions with or without concomitant inflammation in the same anatomical area are more strongly associated with new bone formation than inflammatory lesions. If pure inflammatory lesions precede more advanced inflammatory lesions, these results suggest that there may be a window of opportunity for treatments to prevent new bone formation.
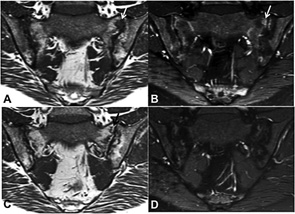
SIJ MRI Inflammation, Other Types of MRI Lesions & Ankyloses
Only two studies have investigated the association between MRI inflammation and other types of MRI lesion and ankyloses in the SIJs of patients with AS.14,15 In a German study, the effect of the TNFα inhibitor etanercept (n=35) and sulfasalazine (n=30) was investigated in a randomized study of one year’s duration.15 A new fat lesion developed in 17 MRI quadrants. New fat lesions did not develop in quadrants without inflammation at baseline (0%). In SIJ quadrants with inflammation at baseline that had resolved at follow-up, new fat lesions appeared in 10.5% of these quadrants. In 2.4% of the quadrants with inflammation at follow-up, concomitant fat lesions had evolved. There was no change in the number of erosions or in ankylosis.
The second study tested the hypothesis that SIJ joint ankylosis develops following repair of erosions and that tissue characterized by fat metaplasia (i.e., fat lesions and/or backfill) is a key intermediary step in this pathway.14 The study comprised 147 patients with AS in an observation cohort of patients who had MRI scans performed at baseline and at two years. Seventy-nine (53.7%) of the patients were treated with a TNFα inhibitor. Multivariate stepwise regression analyses were performed, including baseline demographics and different MRI lesions. Predictors of two-year change in fat lesions were reductions in the concentration of C-reactive protein (p<0.01), reductions in SIJ inflammation score (p<0.03) and reductions in erosion score (p<0.001) over two years. The independent predictors of increase in backfill score were the baseline backfill score (p<0.0001) and reductions in SIJ inflammation score (p<0.003) and erosion score (p<0.0001). New SIJ ankylosis after Year 2 was independently predicted by reductions in erosion score (p<0.002) and backfill score (p<0.02), and an increase in fat score (p<0.002).
Thus, in patients with AS, resolution of SIJ inflammation is associated with development of fat metaplasia (i.e., fat lesions located inside the bone marrow) and backfill located in the excavation of erosions. These fat lesions may represent a repair response after resolution of SIJ inflammation leading to new bone formation.
[Study] results support the hypothesis that erosive bone destruction is followed by repair tissue, which develops into mature bone when inflammation has resolved.
Discussion & Perspectives
Overall, the spine studies support a positive association between inflammation, fat and new bone formation, with the association between fat and new bone being stronger than the association between inflammation and new bone. The diverging results for inflammation may be explained by the different definitions. The weakest association was observed for the vertebral unit definition, which covers inflammation located at any area along both endplates in relation to a disk. In contrast, the associations were stronger for vertebral corner lesions, located in the same area as the syndesmophyte on radiography. Consequently, methodological issues are important to take into consideration when interpreting the results of the spine studies.
In the SIJs, fat metaplasia (i.e., fat lesions in the bone marrow and backfill in the excavation of erosion) seems to be an intermediary step between erosion and new bone formation. However, only one study has been performed, and more studies should be performed. Nevertheless, the results support the hypothesis that erosive bone destruction is followed by repair tissue, which develops into mature bone when inflammation has resolved.
These studies provide important new information on the pathology of AS and the impact of different therapeutics. The studies also provide information on potential predictors for structural damage progression.

Susanne Juhl Pedersen, MD, PhD, is affiliated with the Copenhagen Center for Arthritis Research (COPECARE), Center for Rheumatology and Spine Diseases, Rigshospitalet, Glostrup, University of Copenhagen, Denmark.
References
- Sieper J, Appel H, Braun J, et al. Critical appraisal of assessment of structural damage in ankylosing spondylitis: Implications for treatment outcomes. Arthritis Rheum. 2008 Mar;58(3):649–656.
- Maksymowych W, Pedersen SJ, Ostergaard M, et al. MRI of the spine for detection of new bone formation in ankylosing spondylitis: Does it offer any advantages over radiography. Ann Rheum Dis. 2013 Jun;71(Suppl3):147.
- Spoorenberg A, de Vlam K, van der Linden S, et al. Radiological scoring methods in ankylosing spondylitis. Reliability and change over 1 and 2 years. J Rheumatol. 2004 Jan;31(1):125–132.
- Wanders AJ, Landewe RB, Spoorenberg A, et al. What is the most appropriate radiologic scoring method for ankylosing spondylitis? A comparison of the available methods based on the Outcome Measures in Rheumatology Clinical Trials filter. Arthritis Rheum. 2004 Aug;50(8):2622–2632.
- Ostergaard M, Maksymowych WP, Pedersen SJ, et al. Structural lesions detected by magnetic resonance imaging in the spine of patients with spondyloarthritis—definitions, assessment system, and reference image set. J Rheumatol. 2009 Dec;84(Suppl):18–34.
- Maksymowych WP, Wichuk S, Chiowchanwisawakit P, et al. Development and preliminary validation of the spondyloarthritis research consortium of Canada magnetic resonance imaging sacroiliac joint structural score. J Rheumatol. 2015 Jan;42(1):79–86.
- Baraliakos X, Listing J, Rudwaleit M, et al. The relationship between inflammation and new bone formation in patients with ankylosing spondylitis. Arthritis Res Ther. 2008;10(5):R104.
- Maksymowych WP, Chiowchanwisawakit P, Clare T, et al. Inflammatory lesions of the spine on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis: Evidence of a relationship between inflammation and new bone formation. Arthritis Rheum. 2009 Jan;60(1):93–102.
- Pedersen SJ, Chiowchanwisawakit P, Lambert RG, et al. Resolution of inflammation following treatment of ankylosing spondylitis is associated with new bone formation. J Rheumatol. 2011 Jul;38(7):1349–1354.
- van der Heijde D, Machado P, Braun J, et al. MRI inflammation at the vertebral unit only marginally predicts new syndesmophyte formation: A multilevel analysis in patients with ankylosing spondylitis. Ann Rheum Dis. 2012 Mar;71(3):369–373.
- Baraliakos X, Heldmann F, Callhoff J, et al. Which spinal lesions are associated with new bone formation in patients with ankylosing spondylitis treated with anti-TNF agents? A long-term observational study using MRI and conventional radiography. Ann Rheum Dis. 2014 Oct;73(10):1819–1825.
- Chiowchanwisawakit P, Lambert RG, Conner-Spady B, et al. Focal fat lesions at vertebral corners on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis. Arthritis Rheum. 2011 Aug;63(8):2215–2225.
- Maksymowych WP, Morency N, Conner-Spady B, et al. Suppression of inflammation and effects on new bone formation in ankylosing spondylitis: Evidence for a window of opportunity in disease modification. Ann Rheum Dis. 2013 Jan;72(1):23–28.
- Maksymowych WP, Wichuk S, Chiowchanwisawakit P, Lambert RG, Pedersen SJ. Fat metaplasia and backfill are key intermediaries in the development of sacroiliac joint ankylosis in patients with ankylosing spondylitis. Arthritis Rheumatol. 2014 Nov;66(11):2958–2967.
- Song IH, Hermann KG, Haibel H, et al. Relationship between active inflammatory lesions in the aspine and sacroiliac joints and new development of chronic lesions on whole-body MRI in early axial spondyloarthritis: Results of the ESTHER trial at Week 48. Ann Rheum Dis. 2011 Jul;70(7):1257–1263.