Immune checkpoint inhibitors (ICIs) are at the forefront of advances in cancer therapy and have shown promising results for progression-free survival. Checkpoint signaling pathways, such as cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1), normally regulate the immune response to promote self-tolerance and prevent tissue damage and inflammation.
PD-1 is a co-inhibitory receptor expressed on activated T cells. It “turns off” T cells that would normally destroy cancer cells. Solid tumors have increased expression of PD-1, which is often associated with a worse prognosis. Blockage of PD-1 with ICIs enhances T cell function and tumor lysis. This is the basis of using monoclonal antibodies to PD-1 inhibitors as targeted immunotherapy cancer agents.1,2
PD-1 inhibition not only enhances the immune response to tumor cells but also to normal host tissue. Immune-related adverse events (irAEs) from ICIs occur from self-intolerance with inhibition of checkpoint sites and uncontrolled activity of T cells, leading to a multi-organ inflammatory response that can mimic a rheumatologic condition. irAEs, graded 1–4, can occur in variable frequency at any time during therapy. They have been reported in higher numbers with the use of CTLA4 inhibitors, such as ipilimumab, or combination PD1 inhibitors (e.g., nivolumab, pemrolizumab) but less commonly with nivolumab monotherapy.2,3 Nivolumab (Opdivo) has been approved for melanoma, squamous cell cancer of the head and neck, Hodgkin’s lymphoma, colorectal, renal cell and non-small cell lung cancer.
irAEs can involve any organ system, but myalgias, arthralgias, gastrointestinal, dermatologic, hepatic and endocrine autoimmune effects are those most commonly reported with nivolumab use. Cardiac, pulmonary and ocular toxicities are rare.3-5
Nivolumab and ICIs are being tested for other malignancies, such as ovarian cancer, which is the most lethal of gynecologic malignancies, with a five-year survival rate of 25% for women diagnosed with advanced stage disease despite surgery and chemotherapy. Recurrence is associated with poor prognosis and resistance to standard treatment. Recent research in oncology has shown that ovarian cancers have an increased expression of PD-1 and poorer outcomes; PD-1 inhibition is the basis of current investigations for nivolumab’s use in ovarian cancer.6
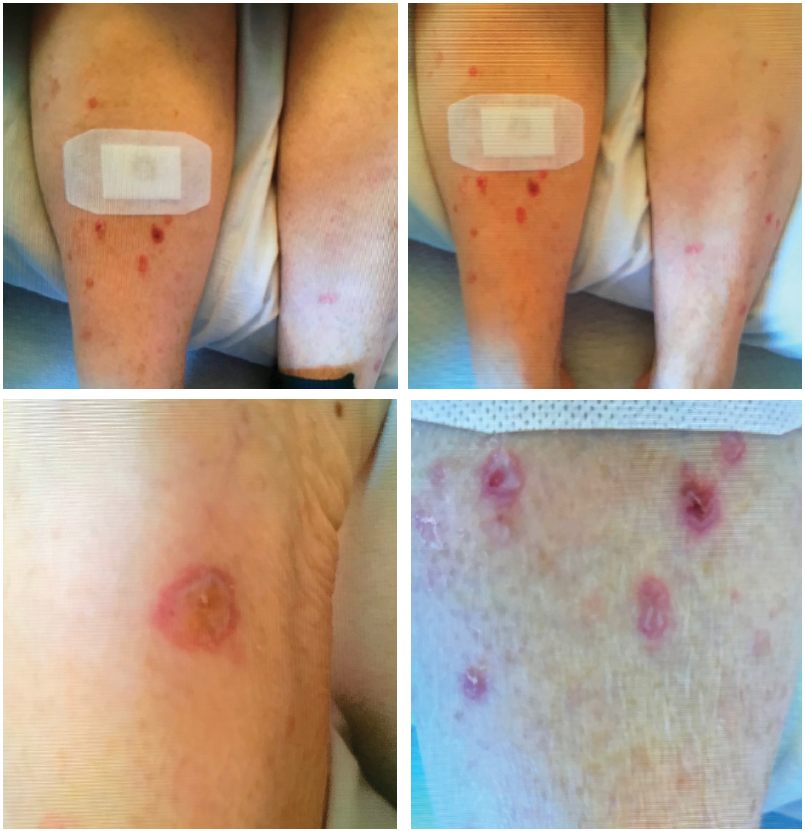
The patient presented with multiple, bullous, round, erythematous plaques on both of her lower, distal extremities. Biopsy showed perivascular inflammatory cell infiltrate concerning for drug eruption, collagen vascular disease or early autoimmune vesiculobullous disorder.
We present the case of a patient with a history of cardiac disease on statin therapy and ovarian cancer treated with nivolumab who developed cardiac, musculoskeletal, respiratory, neurologic, dermatologic and gastrointestinal autoimmune adverse events. This case is unique for several reasons:
- Nivolumab was used as a novel agent for ovarian cancer;
- Its use resulted in multiple autoimmune toxicities, as well as potentially life-threatening manifestations of cardiotoxicity and inflammatory myositis that are rarely reported with its use; and
- Ovarian cancer and statin use itself can lead to autoimmune myositis and needed to be differentiated from nivolumab toxicity.
Case Presentation
A 75-year-old woman with a past medical history of hypertension, osteopenia, coronary artery disease with one stent, hyperlipidemia on 20 mg of atorvastatin and no known personal or family history of rheumatologic disorders was diagnosed with stage 3c, high-grade, serous ovarian cancer in 2014. She was treated with hysterectomy, oophorectomy and multiple cycles of paclitaxel and carboplatin, but the cancer recurred in 2015 with hepatic metastasis. She received further cycles of carboplatin and docetaxel and went into remission.
In 2016, she was being treated with nivolumab to maintain remission at another hospital. A few days after the first treatment, she developed diarrhea and a rash on both lower shins, but no workup or intervention was done at the time.
Two weeks later, the patient received her second cycle of nivolumab, with immediate worsening of the pretibial rash (see Figure 1). Seven days after that infusion, she presented at our institution with generalized fatigue, weakness and syncope. On initial evaluation, she was found to be bradycardic with a heart rate of 33 beats per minute. An electrocardiogram showed third-degree atrioventricular block. Laboratory data were remarkable for troponin levels of 27.3 ng/L (upper limit of normal [ULN] <0.6 ng/L]), creatine kinase (CK) levels of 6,322 IU/L (normal = 42-284 IU/L), CK-MB levels of 250 ng/dL (ULN<5.0 ng/dL), ALT levels of 182 u/L (normal = 4–36 IU/L) and AST levels of 247 u/L (normal = 13–39 IU/L).
The patient became hemodynamically unstable, and an emergent pacemaker was implanted. Her symptoms improved. Echocardiography revealed mild concentric left ventricular hypertrophy, mild mitral regurgitation, mild pulmonary hypertension and an ejection fraction of 55–60%. Cardiac catheterization showed multivessel disease that was stable when compared to results from the prior year. The cardiac catheterization excluded acute ischemic disease as the cause of the heart block, and no stent was placed. She received intravenous fluids. Her CK and troponin levels dropped to 3,000 IU/L and 16 ng/L, respectively. She also developed atrial fibrillation.
While the patient was hospitalized, she developed right eyelid droop and generalized weakness. A neurology assessment confirmed ptosis and 1 mm pupil asymmetry without other neurologic findings. Acetylcholine receptor antibody tests, and brain and spine imaging were negative. The working diagnosis was nerve infarct, aneurysm or myasthenia gravis.
The patient was prepared for discharge with a diagnosis of rhabdomyolysis and ischemia in the setting of cardiac hypoperfusion, and she was scheduled for outpatient cardiology and neurology follow-up. She was taken off the statin.
The patient returned to the hospital one week later with sudden onset shortness of breath, inability to clear phlegm, dysphagia, dysphonia and proximal upper and lower extremity muscle weakness and pain. She was unable to sit up in bed or stand. Prior to this, she had been walking two miles daily for exercise.
Laboratory analysis showed her cardiac and liver markers were still elevated and had actually been rising since her hospital discharge one week earlier: CK of 5,029 IU/L, CK-MB of 275 ng/dL, troponin of 6.2 ng/mL, ALT of 422 u/L and AST of 421 u/L.
At this point, rheumatology was consulted to rule out myositis. The exam was significant for decreased strength of hip flexion and knee extension (2/5); proximal girdle weakness of her upper and lower extremities bilaterally; ptosis of the right eyelid; stridor; pooling of upper airway secretions in the posterior pharynx; coarse breath sounds in both lungs; and multiple, bullous, 0.5–1.5 cm, round, erythematous plaques on both distal lower extremities. Pulse oximetry was 90% at rest, and the patient was started on oxygen therapy.
A chest X-ray showed right basilar atelectasis and a small pleural effusion. On pulmonary evaluation, a computed tomography (CT) scan of the lung did not show interstitial lung disease, alveolitis or pulmonary embolus, but did show mucous collections in the distal airway. Ear, nose and throat evaluation suggested laryngeal and vocal cord edema contributing to stridor. A modified barium swallow was concerning for oropharyngeal dysphagia, and a nasogastric tube had to be inserted for enteral nutrition.
Her aldolase was elevated at 62 u/L (upper limit of normal [ULN] <7.5 units/liter). Autoimmune serologies and specific markers of myositis were negative—including ANA, anti-dsdna, anti-smith, anti-RNP, SSA/SSB, anti-Scl, anti-striational, anti-acetylcholine receptor binding (Ach-Ab), HMG Co-A reductase, anti-Jo, anti-SRP, anti-Mi2 and myositis panel (PL-7, PL-12, MI-2, KU, EJ, OJ). A thyroid panel and antibodies were normal. CA125 markers and CTs of her chest, abdomen and pelvis were negative for recurrence of ovarian cancer. Consultations with the gynecology and oncology departments deemed the cancer in remission.
The dermatologist performed a punch biopsy of the pretibial rash. It showed perivascular inflammatory cell infiltrate with lymphocytes, histiocytes, rare eosinophils, a few plasma cells and neutrophils. The infiltrate subtly obscured the dermo-epidermal junction where there was vacuolar alteration and necrotic keratinocytes within the epidermis. Signs of focal ulceration and subepidermal clefting were evident. A PAS stain was negative for hyphae or basement membrane zone thickening. We were concerned about a drug eruption, manifestation of collagen vascular disease or early autoimmune vesiculobullous disorder.
Nerve conduction studies and electromyography were performed in the right upper and right lower extremities and were consistent with myopathic change of the muscles without suggestion of large fiber polyneuropathy or necrosis. Specifically, there was low amplitude of the right sural sensory response and right tibial motor response and absence of right peroneal F right tibial H reflexes. Skeletal muscle biopsy of the left quadriceps revealed immune-mediated myopathic disorder, identified by a positive HLA Class I immunohistochemistry study, with focal myofiber necrosis/regeneration, mild inflammation and type 2 myofiber atrophy. The findings were consistent with an immune-mediated (inflammatory) myopathy, subtype not specifically identified (see Figure 2).
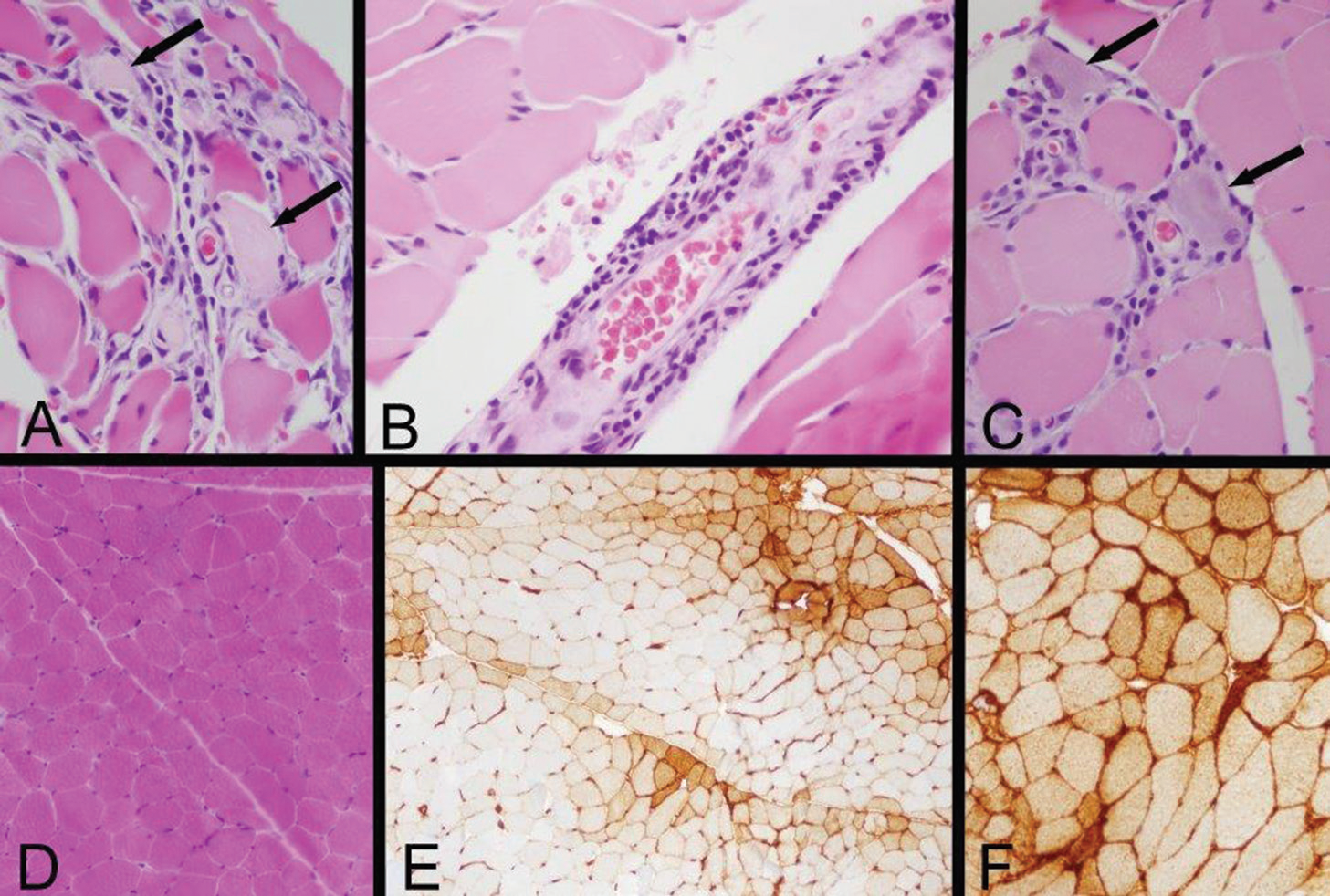
Figure 2: Left Quadriceps Muscle Biopsy
A: Two necrotic myofibers with pale sarcoplasm are identified by arrows in this area of a paraffin hematoxylin and eosin (H&E) section, which also exhibits very mild endomysial lymphocytic inflammation.
B: A mild perimysial perivascular lymphocytic inflammatory infiltrate is present in this region of a paraffin H&E section.
C: Arrows indicate two regenerating myofibers, which are identified by their basophilic cytoplasm and large nuclei with nucleoli, and a small number of lymphocytes infiltrate this zone (paraffin H&E). Inflammation associated with necrotic or regenerating myofibers may be a reactive change rather than evidence of an inflammatory myopathy; the perivascular inflammation provides more convincing evidence of an immune-mediated myopathy.
D: A low-power view of a portion of a cryostat H&E section illustrates that much of the biopsy has no inflammation and no myofiber necrosis or regeneration.
E: Human leucocyte antigen Class ABC or Class I (HLA Class I) immunohistochemistry exhibits labeling of many myofibers, indicated by the brown staining. In this area, there is a somewhat perifascicular concentration of positive myofibers.
F: HLA Class I immunohistochemistry shows strong surface labeling and sarcoplasmic staining of all of the myofibers in this area. Original magnifications: A, B, C—400x; D, E—100x; F—200x
The constellation of findings, temporal association of events and presence of multi-organ involvement raised suspicion for an immunotherapy-mediated toxicity. The patient was started on 1 mg/kg of methylprednisolone immediately after the muscle biopsy. The choice of steroid dosing was based on guidelines for management of the known toxicities of nivolumab (skin, pneumonitis, neurologic) because no guidelines on the management of myositis or cardiotoxicity secondary to nivolumab therapy were known to have been published at the time.
Within two days of starting steroids, the patient demonstrated improvement in her respiratory status and stridor. On Day 3, she was able to sit up without assistance. By Day 7, her dysphagia and dysphonia were significantly improved, and the nasogastric tube was removed. Her dyspnea also improved, and the patient no longer required oxygen for relief. An ultrasound of the diaphragm following the steroid treatment showed appropriate movement with respiration. Her ptosis resolved, and the rash gradually diminished in intensity and size.
By the time of discharge three weeks later, the patient was again able to walk, albeit a few steps. Her lab values had normalized: CK of 126 IU/L, troponin of 0.3 ng/mL, ALT of 95 u/L and AST of 32 u/L. She was discharged to rehab with an oral prednisone taper.
The patient regularly followed up in the rheumatology clinic and made significant progress in her strength. None her symptoms recurred during the prednisone taper, even at doses less than 10 mg. Her CK, troponin, AST and ALT remained normal on serial surveillance. She was restarted on statin therapy without adverse events. Six months later, she was walking without any assistive devices and had regained full functionality of her routine lifestyle.
Discussion & Literature Review
This case is unique in that nivolumab was being used as a novel agent for ovarian cancer. Further, its use led to a unique constellation of inflammatory responses that overlap with autoimmune conditions. The patient developed myositis, heart block, ptosis, dysphagia, dysphonia, rash and respiratory stridor, which are not typically reported and even less likely to manifest concomitantly.
The acuity of symptom onset and temporal association of events, worsening of symptoms following additional doses of nivolumab, muscle and skin biopsy evidence of inflammation, and resolution of symptoms with steroid use raised concern that the patient was having an immune-mediated adverse event from nivolumab.
Myositis: Myalgias and arthralgias are associated with 2–12% of cases of ICI use, but myositis is rarely reported. The initial rheumatologic consultation was to rule out polymyositis, dermatomyositis or statin myopathy in the setting of underlying malignancy, muscle weakness and rash. The association between certain malignancies (e.g., ovarian, breast, lung, gastric, lymphoma) and myositis is well known to rheumatologists. Dermatomyositis (DM) or paraneoplastic necrotizing myopathy (PNM) has been reported with malignancies. Sometimes these are reported as a presenting feature of the malignancy. However, DM or PNM did not explain all of the patient’s symptoms, including the cardiac and neurologic manifestations. Myositis antibodies were negative. The rash was pretibial and atypical for heliotrope or shawl sign, Gottron’s papules, poikiloderma or photosensitivity. The symptoms occurred at a point in time when the patient’s cancer was in remission and did not occur during her initial diagnosis in 2014 or recurrence in 2015.7,8
The relationship between cancer and paraneoplastic myositis is also thought to be mediated through the immune system. Auto-antigen triggers are thought to overlap between cancer cells and myositis cells, in which the immune-mediated destruction of muscle may be a paraneoplastic manifestation of the immune system’s response to the cancer. The use of PD1 inhibitors may further complicate the immune system’s balance through their effect on signaling pathways.9,10
Another consideration for a possible cause of myositis in this patient was statin use, which can cause an immune-mediated necrotizing myopathy. However, the rash and presence of other symptoms were not typical of statin-induced myopathy, and neither was the muscle biopsy report. She was also able to tolerate restarting statin therapy.11,12
Cardiotoxicity: Cardiotoxicity is a rarely reported irAE (<1%), and not well known with nivolumab. It was labeled as rhabdomyolysis during the first admission but prompt recognition is important as cardiac irAE can prove fatal. Most cases of myositis and cardiotoxicity are reported with ipilimumab or nivolumab/ipilimumab combination therapy for melanoma.
Cardiac irAEs include heart failure, cardiomyopathy, heart block, myocardial fibrosis and myocarditis. Autopsy specimens reveal an underlying lymphocytic infiltration with interstitial fibrosis, which may contribute to conduction defects.3,4,13
The etiology of ICP1-induced myocarditis has also been investigated in mice models. Deficiency of PD-1 leads to inflammation of myocytes, formation of antibodies to troponin and cardiotoxicity.14-16
Patients with preexisting cardiac conditions, such as our patient, seem to be at greater risk and need to be carefully screened prior to initiating PD-1 inhibitors. Measuring serial CK markers with each administrative dose of ICI may help monitor development of an inflammatory muscle response in its early stages and prevent further administration of the drug if toxicity is developing. Prompt recognition and steroid initiation may help avoid fatal cardiac outcomes.15
Commonly Reported irAEs: Although myositis and cardiotoxicity were not commonly reported by the drug manufacturers at the time of this patient’s presentation, knowledge of the timing of onset of other known irAEs was helpful in this case to suggest the events were drug related. Dermatologic manifestations from ICI use are the most common irAEs and usually occur in the first few weeks—as occurred in this patient, and which worsened with repetitive dosing.3 Skin toxicity varies in presentation from rash to pruritus to vitiligo. The histology differs, but this patient’s biopsy did show inflammation and perivascular infiltrates. PD-1 preserves epidermal integrity during inflammatory skin reactions, and deficiency in mice models has shown lupus-like inflammatory syndrome with proliferative glomerulonephritis and arthritis. Histology reveals cytotoxic skin eruption with accumulation of CD8 T cells at the dermo-epidermal junction. Severe toxicity, such as Steven-Johnson or toxic epidermal necrolysis, can also rarely occur.17,18
Gastrointestinal symptoms of diarrhea and enterocolitis commonly occur after ICI initiation. The patient’s liver transaminitis can be attributed to muscle damage or hepatitis. Drug-induced liver injury (DILI) has been reported as an irAE in approximately 1–5% of patients six to 12 weeks after starting ICIs. Liver biopsies show a predominant CD-8 positive inflammatory infiltrate indistinguishable from autoimmune hepatitis. Systemic and topical steroids, such as budesonide, are proposed treatments.3,19,20
The patient’s pulmonary presentation was acute and life threatening. The differential was broad, including pneumonitis, interstitial lung disease related to myositis, myasthenia-related diaphragmatic failure or upper airway obstruction secondary to mucous and edema. Pneumonitis and lung fibrosis are reported irAEs, and more likely to occur in patients with lung cancer being treated with an ICI.3,5
Renal injury, uveitis, hematologic abnormalities, hypophysitis, thyroid dysfunction and other autoimmune endocrine abnormalities, such as type 1 diabetes, have also been commonly reported.2-4
The presence of ptosis, respiratory issues, dysphagia, dysphonia and muscle weakness also suggested the possibility of myasthenia gravis. Other reported neurologic irAEs include Guillain-Barré syndrome, encephalopathy, and Bell’s palsy. Interestingly, a case has been reported of a patient with baseline, low-titer positive acetylcholine receptor binding antibody who was treated with nivolumab for melanoma and developed myasthenia gravis.21
Role of Antibodies: The value of antibody testing is important to understand and predict the occurrence of irAEs from ICIs use. In another case, a patient with papillary transitional cell cancer and preexisting anti-striated muscle antibody developed severe polymyositis following treatment with nivolumab and ipilimumab.
These authors concluded that subclinical preexisting autoantibodies predispose patients to develop autoimmune conditions when on ICIs. The relationship between antibody preexistence or formation is an important area of further investigation. This has been difficult to assess, because PD-1 inhibitors are not generally tested in patients who have baseline autoimmune conditions and require immunosuppression. On the other hand, it may be important to screen patients for autoimmune conditions and determine their susceptibility to developing severe irAEs before initiating treatment with ICIs.21-24
Although patients with certain antibodies may be predisposed to develop an irAE from ICI use, this was not the case for our patient or other reported cases. Another case report actually suggested the opposite relationship—a patient with melanoma with no preexisting antibodies was treated with ipilimumab and later developed anti-TSH antibodies and Graves’ opthalmopathy.24 This signifies that there are other underlying immune-mediated mechanisms leading to activation of multiple self-intolerant pathways and manifesting as overlapping autoinflammatory conditions. For example, PD-1 has higher expression in cardiac tissues than in other organs. The basis of why specific organs are affected in certain patients but not in others with the same ICI use needs to be elucidated.14,20,21 Answers to these questions may help screen which patients are candidates for immunotherapy or predict which patients may be at risk for developing an irAE.
Long-Term Treatment & Monitoring
Discontinuation of the drug was not enough for our patient; she experienced cardiac and respiratory compromise. Steroids may have saved her life, but she developed secondary side effects of weight gain and osteoporosis.
No data exist on the appropriate tapering of steroids for irAEs. Some ongoing research suggests the use of immune modulatory medications, such as infliximab, tumor necrosis factor alpha inhibitors, plasmapheresis or intravenous immunoglobulin for the management and long-term suppression of irAEs or to be used alongside ICI therapy to blunt the inflammatory response at the onset. This needs to be further studied for each of the rapidly growing number of ICIs.3,5,25
This case also poses a difficult question for patients and oncologists regarding how they will manage ICI use once an irAE occurs. When an adverse event occurs during ICI therapy, it is important to ascertain if it is from disease progression, an unrelated event or treatment-related irAE. Characterizing these toxicities, even if uncommon, is important.
Management of irAEs is under intense investigation at this point, but general guidelines are being developed. No clear data exist on when to stop ICI therapy or when to reintroduce an ICI once the autoimmune process is managed with steroids for control of responsive cancers.
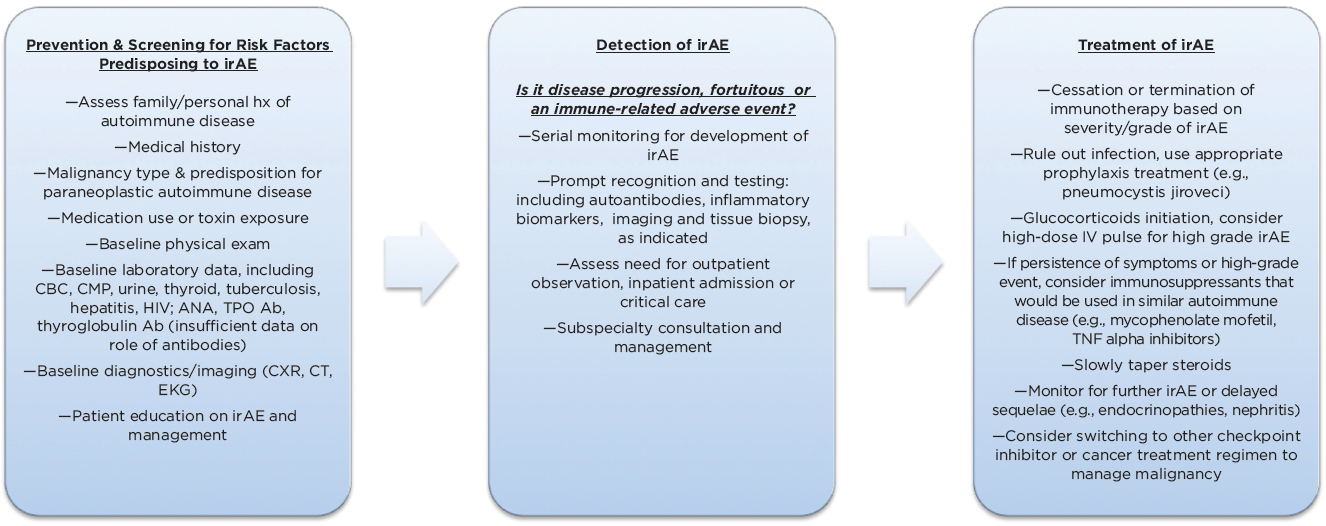
(click for larger image) Figure 3: Management Algorithm for Immune-Related Adverse Events (irAEs) Associated with Checkpoint Inhibitor Use (Adapted)
In the long term, it is unclear how the patient’s underlying immunologic makeup is affected after the use of ICIs or after the development of an autoimmune condition. Further, predictive biomarkers or antibodies that would help identify which patients and organ systems are at risk for immune-mediated toxicity would help target cancer therapy (see Figure 3).2,3,5,25
Immune-related adverse events can involve any organ system, but myalgias, arthralgias, gastrointestinal, dermatologic, hepatic & endocrine autoimmune effects are those most commonly reported with nivolumab use.
Conclusion
This patient had a cardiac history, was on statin therapy and had ovarian cancer treated with the PD-1 inhibitor nivolumab. She developed life-threatening immune-mediated adverse events, including cardiac toxicity, myositis, myasthenia-like syndrome, pulmonary compromise, rash and gastrointestinal toxicity mimicking auto-inflammatory rheumatologic conditions. Immune checkpoint inhibitors have high response rates and overall better tolerance compared with chemotherapy. However, with their increased and broadening uses, it is becoming evident they have a unique inflammatory toxicity innate to the way they affect the immune system with variable presentations. It will be especially important to monitor malignancies often associated with paraneoplastic autoimmune responses themselves in trying to differentiate toxicity of the treatment from manifestation of the cancer.
The questions raised and the management of affected patients requires an interdisciplinary approach. Rheumatologists, oncologists and all subspecialties will need to become familiar and adept at rapidly recognizing and managing irAEs because checkpoint inhibitor use is becoming increasingly prevalent and will likely become the future of cancer therapy.
Priya Chokshi, MD, is a second-year rheumatology fellow at NYU Winthrop Hospital in New York and will be pursuing a career in academic rheumatology.
Roberta Seidman, MD, is an associate professor of pathology at Stony Brook Medical Center in New York. She is board certified in both neuropathology and neurology and has more than 30 years’ experience in evaluation of nerve and muscle biopsies and brain autopsy specimens.
Noah Levit, PhD, MD, is a third-year postgraduate dermatology resident at the University of Colorado, Anschutz Medical Campus. His interests include cutaneous toxicity, oncodermatology, and improving access to dermatologic care for cancer patients
Steven Carsons, MD, is chief of the Division of Rheumatology, Allergy and Immunology and program director of the rheumatology fellowship program at NYU Winthrop Hospital in New York.
Acknowledgments
The authors would like to thank the following: Elise Belilos, MD, Rheumatology Division, NYU Winthrop Hospital; Gary Rosenblum, MD, Rheumatology Division, NYU Winthrop Hospital; Kristina Belostocki, MD, Rheumatology Division, NYU Winthrop Hospital; Eun Ji Kwon, MD, Dermpath Diagnostics Pathology Associates
References
- McDermott D, Atkins M. PD-1 as a potential target in cancer therapy. Cancer Med. 2013 Oct;2(5):662–673.
- Suarez-Almazor M, Kim S, Abdel-Wahab N, Diab A. Review: Immune-related adverse events with use of checkpoint inhibitors for immunotherapy of cancer. Arthritis Rheumatol. 2017 Apr;69(4):687–699.
- Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015 Dec;26(12):2375–2391.
- Zimmer L, Goldinger SM, Hofmann L, et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer. 2016 Jun;60:210–225.
- Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. 2016 Mar;44:51–60.
- Gaillard S, Secord A, Monk B. The role of immune checkpoint inhibition in the treatment of ovarian cancer. Gynecol Oncol Res Pract. 2016 Nov 24;3:11.
- Min KJ, Ouh YT, Hong HR, et al. Muscle weakness and myalgia as the initial presentation of serous ovarian carcinoma: A case report. J Ovarian Res. 2014 Apr 23;7:43.
- Whitmore SE, Rosenshein NB, Provost TT. Ovarian cancer in patients with dermatomyositis. Medicine (Baltimore). 1994 May;73(3):153–160.
- Levine SM. Cancer and myositis: New insights into an old association. Curr Opin Rheumatol. 2006 Nov;18(6):620–624.
- Sigurgeirsson B, Lindelöf B, Edhag O, Allander E. Risk of cancer in patients with dermatomyositis or polymyositis. N Engl J Med. 1992 Feb 6;326(6):363–367.
- Mammen AL. Statin-associated autoimmune myopathy. N Engl J Med. 2016 Feb 18;374(7):664–669.
- Hansen KE, Hildebrand JP, Ferguson EE, Stein JH. Outcomes in 45 patients with statin-associated myopathy. Arch Intern Med. 2005 Dec 12–26;165(22):2671–2676.
- Heinzerling L, Ott PA, Hodi FS, et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer. 2016 Aug 16;4(50):1–11.
- Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016 Nov 3;375(18):1749–1755.
- Tarrio ML, Grabie N, Bu DX, et al. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J Immunol. 2012 May 15;188(10):4876–4884.
- Lucas JA, Menke J, Rabacal WA, et al. Programmed death ligand 1 regulates a critical checkpoint for autoimmune myocarditis and pneumonitis in MRL mice. J Immunol. 2008 Aug 15;181(4):2513–2521.
- Goldinger SM, Stieger P, Meier B, et al. Cytotoxic cutaneous adverse drug reactions during anti-PD-1 therapy. Clin Cancer Res. 2016 Aug 15;22(16):4023–4029.
- Lo JA, Fisher DE, Flaherty KT. Prognostic significance of cutaneous adverse events associated with pemrolizumab therapy. JAMA Oncol. 2015 Dec;1(9):1340–1341.
- Ziemer M, Koukoulioti E, Beyer S, et al. Managing immune checkpoint-inhibitor-induced severe autoimmune-like hepatitis by liver-directed topical steroids. J Hepatol. 2017 Mar;66(3):657–665.
- Johncilla M, Misdraji J, Pratt DS, et al. Ipiimumab-associated hepatitis: Imaging and clinicopathologic characterization in a series of 11 cases. Am J Surg Pathol. 2015 Aug;39:1075–1084.
- Kimura T, Fukushima S, Miyashita A, et al. Myasthenic crisis and polymyositis induced by one dose of nivolumab. Cancer Sci. 2016 Jul;107(7):1055–1058.
- Shirai T, Sano T, Kamijo F, et al. Acetylcholine receptor binding antibody-associated myasthenia gravis and rhabdomyolysis induced by nivolumab in a patient withmelanoma. Japanese J Clin Onc. 2016 Jan;46(1):86–88.