A 53-year-old female presented to the clinic for severe polyarticular joint pain and was found to have a seronegative inflammatory arthritis. Six months before, she had completed 10 months of treatment for stage IV metastatic melanoma with the immune checkpoint inhibitors, nivolumab and ipilimumab, achieving complete remission of her cancer.
She said that throughout her course of immunotherapy treatment she had diffuse aching in her large joints, especially on the days immediately after her infusions, as well as dry eyes and dry mouth. Her course was complicated by several adverse events, including rash and colitis, for which she received prednisone ranging from 20–60 mg daily. By the time she had completed her last infusion of nivolumab, the joint pain and stiffness had spread to the small joints of her hands.
After our evaluation showed synovitis in the small joints of the hands, wrists and knees, she was started on methotrexate and prednisone, with improvement in her arthritis disease activity over several months. Her sicca syndrome remains active, requiring frequent use of replacement eye drops. Her melanoma remains in remission.
Immune Checkpoint Inhibitors
Immune checkpoint inhibitors (ICIs) have dramatically changed the face of cancer treatment, and in 2013, Science magazine deemed cancer immunotherapy the “breakthrough of the year.”1 ICIs have shown clinical benefit for the treatment of advanced cancers, including melanoma, non-small/small cell lung cancer, renal cell carcinoma, Hodgkin’s lymphoma, head and neck cancers, and urothelial cell carcinoma.
Numerous clinical trials are underway to evaluate ICI efficacy in a variety of other tumor types and in earlier stages of disease. The side effects of ICIs are related to their mechanism of action. ICIs cause nonspecific immune activation, which can target non-tumor tissues, thus leading to immune-related adverse events (irAEs). irAEs can resemble various rheumatic diseases, such as inflammatory arthritis and sicca syndrome, but also include diverse manifestations, such as rash, gastrointestinal (GI) disturbance and endocrinopathies. As indications for immune checkpoint blockade expand and ICIs are used in combination, it becomes increasingly important for rheumatologists and rheumatology professionals to recognize irAEs, their connection to cancer immunotherapy and how to treat these events appropriately.
Timeline of Immunotherapies, Mechanisms of Action
Cancer immunotherapy has shifted the current management strategy in advanced cancer from conventional chemotherapy approaches to using endogenous immune cells to target cancer. ICIs are a novel class of immunotherapies, first approved by the FDA in 2011. Several drugs are currently on the market, including ipilimumab, nivolumab, pembrolizumab and atezolizumab; many more ICIs are in clinical trials (see Table 1).
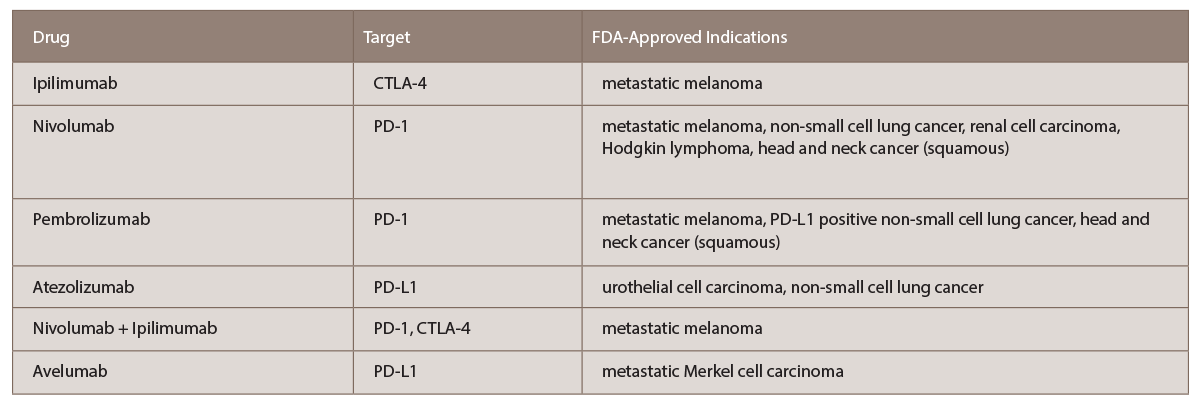
(click for larger image) TABLE 1: List of current FDA-approved immune checkpoint inhibitors with targets and indications (per www.fda.gov).
The idea of using the immune system to fight cancer is more than 120 years old. William B. Coley used a mixture of Streptococcus pyogenes and Bacillus prodigiosus, later referred to as Coley’s toxins, to treat a large inoperable malignant sarcoma, achieving cure.2 The concept of immune system surveillance for the suppression of tumor formation was introduced in 1909 by Paul Ehrlich, not fully gaining acceptance until the early 2000s. Various immunotherapies were tried over the next century, with some success, including interleukin (IL) 2 infusions for treatment of metastatic melanoma and anti-cancer vaccines.
The critical discovery of the cytotoxic T-lymphocyte antigen 4 (CTLA-4) receptor on the surface of T cells occurred in 1986, and 11 years later, antibodies to this receptor were shown to eradicate tumors in mice.1 It was then demonstrated that anti-CTLA-4 antibodies extended life by nearly four months in patients with advanced metastatic melanoma.3 This was the first FDA-approved ICI, subsequently named ipilimumab, an IgG monoclonal antibody to CTLA-4.4
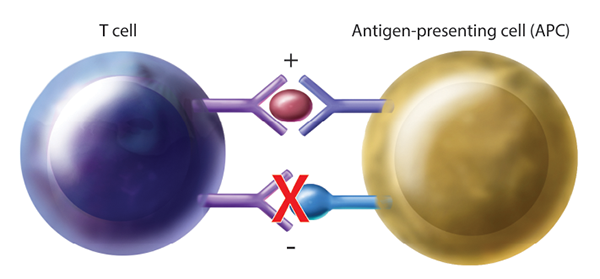
Figure 1: Generalized Mechanism of T Cell Activation Through Immune Checkpoint Blockade. By blocking inhibitory pathways, T cell activation is enhanced, allowing for cancer surveillance and targeted killing. APC=antigen-presenting cell.
Brook Coppola
CTLA-4 is a receptor on T cells that, when engaged, inhibits T cell activation. By blocking this inhibitory receptor with CTLA-4 antibodies, T cell activity is upregulated, thus increasing tumor cell targeting and killing (see Figure 1). It is important to note that the activation of T cells is not specific to tumor antigens, thus nonspecific T cell activation may lead to irAEs.5
It is useful to consider the action of abatacept, a fusion protein of the extracellular domain of CTLA-4 and the Fc region of IgG1, which acts in an opposite way of ICIs, by promoting the negative costimulation of T cells.
Monoclonal antibodies to a second major immune checkpoint involved in cancer regulation were in production by the late 2000s. It was noted that programmed cell death receptor ligands (PD-L1), typically expressed on antigen presenting cells, were upregulated in many human cancers, and PD-1 receptors were highly expressed on tumor infiltrating T cells.6 Blocking PD-1 receptors using monoclonal antibodies, including nivolumab and pembrolizumab, also upregulates T cell activation.7 Atezolizumab, directed against the programmed cell death ligand (PD‑L1), has also been approved for treatment of urothelial cell carcinoma and non-small cell lung cancer with similar mechanism.8
Immune-Related Adverse Events
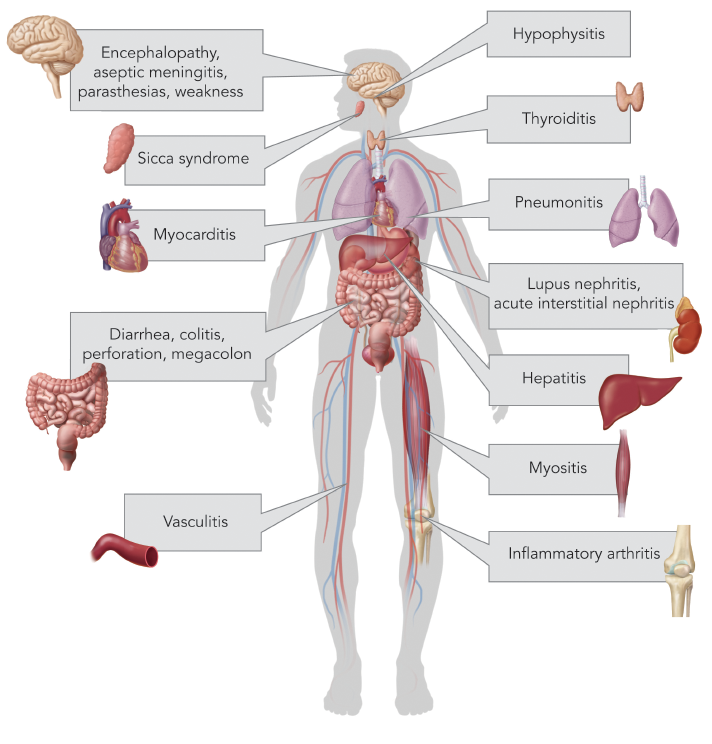
(click for larger image) Figure 2: Common Immune-Related Adverse Events by Organ System
Brook Coppola
Any immune system-mediated tissue damage secondary to ICIs is known as an irAE. irAEs vary in severity and can involve any organ system, but most commonly affect the skin, gastrointestinal tract, endocrine glands such as the thyroid and pituitary, and less commonly the lungs (see Figure 2).9 Rare adverse events are being increasingly recognized, as in a recently published report of two patients who developed fatal myocarditis after treatment with ipilimumab and nivolumab.10
Development of irAEs typically occurs within several weeks of beginning immunotherapy. As noted in clinical trials, the majority of high-grade irAEs related to PD-1 inhibitors occur during the first 12–14 weeks of treatment.11 Combination therapy with nivolumab plus ipilimumab may result in irAE development earlier and more frequently than with monotherapy. This has ranged from skin manifestations at two weeks, GI symptoms at seven weeks, and hepatic and endocrine dysfunction between nine and 12 weeks.12,13
irAEs from ICIs are increasingly recognized as unique entities mimicking traditional rheumatic diseases.14 In a case series of 13 patients treated with nivolumab and/or ipilimumab, nine developed an inflammatory arthritis, and four developed sicca syndrome. The arthritis patients reported in this series were negative for rheumatoid factor and anti-cyclic citrullinated peptide antibodies. In the case of sicca syndrome, dry mouth tended to be more severe than dry eyes.15 Biopsy-proved giant cell arteritis and lupus nephritis have also been reported.16,17 Inflammatory muscle disease has been noted rarely with descriptions of both polymyositis causing significant respiratory muscle weakness and classic dermatomyositis with V-sign and heliotrope rash.
A severe case of dermatomyositis related to ipilimumab therapy for metastatic melanoma has been reported with initial resolution of signs/symptoms on steroids and discontinuation of ICI therapy.18 Interestingly, when the patient’s cancer recurred, she was again treated with ipilimumab, with prompt return of dermatomyositis.
Dermatologic irAEs may be mild to severe. According to a recent meta-analysis, development of a rash with ipilimumab is fairly common, with mild cases occurring in about 24% of patients and high-grade rashes occurring in only 2%.19 The rashes are typically erythematous macules and papules and were not found to be dose dependent. Vitiligo and pruritus have also been seen with use of ipilimumab.
Gastrointestinal irAEs include colitis, diarrhea, obstruction, perforation and toxic megacolon.11 Diarrhea is most pronounced in combination therapy. Similarly, hepatotoxicity is common and when it is severe, can result in AST, ALT and bilirubin levels greater than 20 times the upper limit of normal, with fulminant hepatic failure.
Endocrinopathies related to ICIs may affect the thyroid gland, manifesting as both hypo- and hyperthyroidism, or affect the pituitary gland as hypophysitis.20 Thyroid dysfunction is more often associated with PD-1 inhibitors than ipilimumab; whereas, hypophysitis is most often attributed to the latter. Endocrine dysfunction does not typically resolve due to permanent damage of the glands. Most forms of hypophysitis present as adrenocorticotropic hormone (ACTH) deficiency.
Neurologic irAEs are less frequently reported and include paresthesia, unilateral or bilateral weakness, and altered sensation.21 Aseptic meningitis, encephalopathy, seizures, transverse myelitis, acute and chronic inflammatory demyelinating polyneuropathy, and myasthenia gravis-like syndromes may also occur.22
Pneumonitis is found in less than 5% of patients, ranging from dyspnea to hypoxic respiratory failure, and more commonly associated with combination ICI therapy.23
Evaluation & Management
The role of the rheumatologist will take on growing importance as immunotherapies become more common as standard treatment of cancer and when used earlier in the disease course. Underlying antecedent rheumatic disease should be excluded prior to associating signs and symptoms with immunotherapy. ICIs can worsen previously controlled autoimmune disease, so this is a consideration when evaluating patients. Increased awareness of inflammatory arthritis, as well as other rheumatic manifestations, as an adverse association with cancer immunotherapies is imperative for making the diagnosis. An understanding that more than one irAE is possible within the same patient is also important.
Clinical trials for ICIs may have under-reported rheumatic irAEs due to lack of sign and symptom recognition and standardization for their recording.24 Arthritis has not been consistently reported across these clinical trials, in part, perhaps because of the coding of these events using different terms (e.g., arthralgia, joint effusion, etc.). Therefore, current prevalence estimates of rheumatic irAEs in response to ICI therapy are likely underestimates.
Standard treatment algorithms exist for common symptoms related to ICIs based on manufacturer recommendations.21 Treatment algorithms are based on the severity of symptoms, but in the case of rheumatic disease, treatment often needs to be tailored to the individual.25 The general strategy for evaluation and management of irAEs includes a thorough evaluation for infection. Mild irAEs may be self-limited, while more severe reactions have been generally steroid responsive, albeit with potentially high dosage requirements.15
As for treatment, we propose treating mild articular symptoms with NSAIDs and localized intraarticular injections. If symptoms do not improve or resolve within four to six weeks of treatment, escalation to include low-dose prednisone and possibly holding the ICI should be considered. If symptoms remain severe, consideration of holding the ICI should be given with use of high-dose prednisone (at least 1 mg/kg/day). Once symptom control is attained, steroids should be tapered slowly, over two to four weeks—if not longer.
In severe cases, conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) in addition to TNF inhibitors may be necessary. Shared decision making—among the patient, oncologist and rheumatologist—is crucial to successful treatment. Although csDMARDs have less inherent risk of tumor progression and potential to interfere with immunotherapy, their onset of action is slow. In contrast, and although not approved for this indication, TNF inhibitors may be considered in some circumstances to reduce inflammatory symptoms and improve quality of life more quickly, particularly for patients with lack of response to the ICI, those who have truncated life expectancies, and in patients who do not respond to csDMARDs and/or require prolonged higher dose corticosteroids.
Moreover, there is experience using TNF inhibitors to treat other irAEs, including colitis.26 Recent large observational studies have demonstrated that TNF inhibitors are not associated with increased risk of de novo melanoma and lymphoma development when used to treat inflammatory arthritis.27,28 Little is known, however, about the long-term effects of irAE treatment in patients with advanced cancer. The risk for tumor propagation or impaired cancer response is theoretically possible with biologic agents, such as TNF inhibitors and abatacept.25
It’s important to note that little research exists analyzing use of ICIs in patients with preexisting autoimmune disease because these patients were excluded from original trials. However, preexisting autoimmune diseases should not be an absolute contraindication to ICIs. A careful assessment of disease activity is important prior to starting immunotherapy because of the risk of potential flare.
In a study of 52 patients with autoimmune disease treated with ipilimumab, 20 (38%) had a flare of their disease, including seven of 13 with rheumatoid arthritis, three of three with polymyalgia rheumatica, two of two with Sjögren’s syndrome and three of eight with psoriasis.29 Flares tended to occur in those taking immunosuppressive therapy and with evidence of ongoing autoimmune disease activity at the time of ICI initiation. Only two patients (4%) discontinued their ICI due to severe flare.
irAEs from ICIs are increasingly recognized as unique entities mimicking traditional rheumatic diseases.
Similarly, in a study of 30 patients who received ipilimumab for advanced melanoma with preexisting autoimmune disease (including six patients with RA, five patients with psoriasis, two patients with lupus), half had neither autoimmune flares nor new irAEs.30 However, 10 patients (33%) experienced moderate to severe irAEs; all were successfully treated with corticosteroids. In most cases, disease flares were managed with low-dose corticosteroids ranging from 5 to 30 mg daily. One patient with RA developed severe joint pains after ICI treatment with concurrent hypophysitis and required IV methylprednisolone at 1 mg/kg/day. Infliximab was used to treat two cases of colitis that arose in one patient with preexisting ulcerative colitis and one patient with inflammatory arthritis.
Conclusion
Cancer therapy is rapidly evolving, and manipulating the immune system plays a critical role. The rheumatologist will be faced with new clinical entities, mimicking common autoimmune diseases, and will need to be prepared to rapidly diagnose and treat these irAEs. Preexisting autoimmune diseases were theoretically felt to be a contraindication for use of ICIs, but observational studies have shown successful use in these patients. Early recognition and treatment of these immunologic adverse events will allow for improved outcomes and quality of life for patients previously faced with rapidly fatal disease. Steroids are a mainstay of therapy for the treatment of arthritis, potentially used at higher doses than is typical and for an extended duration. If clinical response is not achieved, csDMARDs and TNF inhibitors may be necessary. Management decisions should always be made in concert with the oncologist.
 Dana Direnzo, MD, is a fellow in the Division of Rheumatology at Johns Hopkins School of Medicine in Baltimore.
Dana Direnzo, MD, is a fellow in the Division of Rheumatology at Johns Hopkins School of Medicine in Baltimore.
 Ami Shah, MD, MHS, is an associate professor of medicine in Division of Rheumatology at Johns Hopkins School of Medicine in Baltimore. A primary research interest for Dr. Shah is the relationship between autoimmune disease and cancer, with a focus on scleroderma.
Ami Shah, MD, MHS, is an associate professor of medicine in Division of Rheumatology at Johns Hopkins School of Medicine in Baltimore. A primary research interest for Dr. Shah is the relationship between autoimmune disease and cancer, with a focus on scleroderma.
 Clifton O. Bingham III, MD, is a professor of medicine in the Division of Rheumatology at Johns Hopkins School of Medicine in Baltimore. He has an extensive background in patient-centered research, clinical trials and translational research in rheumatoid arthritis.
Clifton O. Bingham III, MD, is a professor of medicine in the Division of Rheumatology at Johns Hopkins School of Medicine in Baltimore. He has an extensive background in patient-centered research, clinical trials and translational research in rheumatoid arthritis.
 Laura C. Cappelli, MD, MHS, is an instructor of medicine in the Division of Rheumatology at Johns Hopkins School of Medicine in Baltimore. One of her main clinical and research interests is evaluation and management of rheumatic immune-related adverse events.
Laura C. Cappelli, MD, MHS, is an instructor of medicine in the Division of Rheumatology at Johns Hopkins School of Medicine in Baltimore. One of her main clinical and research interests is evaluation and management of rheumatic immune-related adverse events.
References
- Couzin-Frankel J. Cancer Immunotherapy. Science. 2013 Dec 20;342(6165):1432–1433. doi:10.1126/science.342.6165.1432.
- Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin Orthop Relat Res. 1991 Jan;(262):3–11.
- Hodi FS, O’Day JS, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. New Engl J Med. 2010 Aug 19;363(8):711–723.
- Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse Events Associated with Immune Checkpoint Blockade in Patients with Cancer: A Systematic Review of Case Reports. Nishikawa H, ed. PLoS One. 2016 Jul 29;11(7):e0160221.
- Azoury SC, Straughan DM, Shukla V. Immune checkpoint inhibitors for cancer therapy: Clinical efficacy and safety. Curr Cancer Drug Targets. 2015;15(6):452–462.
- Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–461.
- La-Beck NM, Jean GW, Huynh C, et al. Immune checkpoint inhibitors: New insights and current place in cancer therapy. Pharmacotherapy. 2015 Oct;35(10):963–976.
- Inman BA, Longo TA, Ramalingam S, Harrison MR. Atezolizumab: A PD-L1 blocking antibody for bladder cancer. Clin Cancer Res. 2016 Nov 30. doi: 10.1158/1078-0432.CCR-16-1417.
- Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur J Cancer. 2016;54:139–148.
- Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016 Nov 3;375(18):1749–1755.
- Weber JS, Postow M, Lao CD, Schadendorf D. Management of adverse events following treatment with anti-programmed death-1 agents. Oncologist. 2016 Oct;21(10):1230–1240.
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015 May 21;372(21):2006–2017.
- Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016 Nov;17(11):1558–1568.
- Cappelli LC, Shah AA, Bingham CO. Cancer immunotherapy-induced rheumatic diseases emerge as new clinical entities. RMD Open. 2016 Sep 28;2(2):e000321.
- Cappelli LC, Gutierrez AK, Baer AN, et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis. 2017 Jan;76(1):43–50.
- Fadel F, El Karoui K, Knebelmann B. Anti-CTLA4 antibody-induced lupus nephritis. N Engl J Med. 2009;361(2):211–212.
- Goldstein BL, Gedmintas L, Todd DJ. Drug-associated polymyalgia rheumatica/giant cell arteritis occurring in two patients after treatment with ipilimumab, an antagonist of CTLA-4. Arthritis Rheumatol. 2014 Mar;66(3):768–769.
- Sheik Ali S, Goddard AL, Luke JJ, et al. Drug-associated dermatomyositis following ipilimumab therapy: A novel immune-mediated adverse event associated with cytotoxic T-lymphocyte antigen 4 blockade. JAMA Dermatol. 2015 Feb;151(2):195–199.
- Minkis K, Garden BC, Wu S, et al. The risk of rash associated with ipilimumab in patients with cancer: A systematic review of the literature and meta-analysis. J Am Acad Dermatol. 2013 Sep;69(3):e121–e128.
- Bourke JM, O’Sullivan M, Khattak MA. Management of adverse events related to new cancer immunotherapy (immune checkpoint inhibitors). Med J Aust. 2016 Nov 7;205(9):418–424.
- Food and Drug Administration. Approved risk evaluation and mitigation strategy (REMS).
- Stone JB, DeAngelis LM. Cancer-treatment-induced neurotoxicity—focus on newer treatments. Nat Rev Clin Oncol. 2016 Feb;13(2):92–105.
- Tabchi S, Messier C, Blais N. Immune-mediated respiratory adverse events of checkpoint inhibitors. Curr Opin Oncol. 2016 Jul;28(4):269–277.
- Woodworth T, Furst DE, Alten R, et al. Standardizing assessment and reporting of adverse effects in rheumatology clinical trials II: The Rheumatology Common Toxicity Criteria v.2.0. J Rheumatol. 2007 Jun;34(6):1401–1414.
- Cappelli LC, Shah AA, Bingham CO 3rd. Immune-related adverse effects of cancer immunotherapy—Implications for rheumatology. Rheum Dis Clin North Am. 2017 Feb;43(1):65–78.
- Cheng R, Cooper A, Kench J, et al. Ipilimumab-induced toxicities and the gastroenterologist. J Gastroenterol Hepatol. 2015 Apr;30(4):657–666.
- Mercer LK, Askling J, Raaschou P, et al. Risk of invasive melanoma in patients with rheumatoid arthritis treated with biologics: Results from a collaborative project of 11 European biologic registers. Ann Rheum Dis. 2017 Feb;76(2):386–391.
- Mercer LK, Galloway JB, Lunt M, et al. Risk of lymphoma in patients exposed to antitumour necrosis factor therapy: Results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann Rheum Dis. 2017 Mar;76(3):497–503.
- Menzies AM, Johnson DB, Ramanujam S, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol. 2016 Sep 29. pii: mdw443.
- Johnson DB, Sullivan RJ, Ott PA, et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol. 2016 Feb;2(2):234–240.