Endocrinopathies related to ICIs may affect the thyroid gland, manifesting as both hypo- and hyperthyroidism, or affect the pituitary gland as hypophysitis.20 Thyroid dysfunction is more often associated with PD-1 inhibitors than ipilimumab; whereas, hypophysitis is most often attributed to the latter. Endocrine dysfunction does not typically resolve due to permanent damage of the glands. Most forms of hypophysitis present as adrenocorticotropic hormone (ACTH) deficiency.
Neurologic irAEs are less frequently reported and include paresthesia, unilateral or bilateral weakness, and altered sensation.21 Aseptic meningitis, encephalopathy, seizures, transverse myelitis, acute and chronic inflammatory demyelinating polyneuropathy, and myasthenia gravis-like syndromes may also occur.22
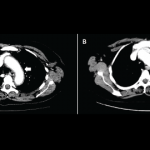
Pneumonitis is found in less than 5% of patients, ranging from dyspnea to hypoxic respiratory failure, and more commonly associated with combination ICI therapy.23
Evaluation & Management
The role of the rheumatologist will take on growing importance as immunotherapies become more common as standard treatment of cancer and when used earlier in the disease course. Underlying antecedent rheumatic disease should be excluded prior to associating signs and symptoms with immunotherapy. ICIs can worsen previously controlled autoimmune disease, so this is a consideration when evaluating patients. Increased awareness of inflammatory arthritis, as well as other rheumatic manifestations, as an adverse association with cancer immunotherapies is imperative for making the diagnosis. An understanding that more than one irAE is possible within the same patient is also important.
Clinical trials for ICIs may have under-reported rheumatic irAEs due to lack of sign and symptom recognition and standardization for their recording.24 Arthritis has not been consistently reported across these clinical trials, in part, perhaps because of the coding of these events using different terms (e.g., arthralgia, joint effusion, etc.). Therefore, current prevalence estimates of rheumatic irAEs in response to ICI therapy are likely underestimates.
Standard treatment algorithms exist for common symptoms related to ICIs based on manufacturer recommendations.21 Treatment algorithms are based on the severity of symptoms, but in the case of rheumatic disease, treatment often needs to be tailored to the individual.25 The general strategy for evaluation and management of irAEs includes a thorough evaluation for infection. Mild irAEs may be self-limited, while more severe reactions have been generally steroid responsive, albeit with potentially high dosage requirements.15
As for treatment, we propose treating mild articular symptoms with NSAIDs and localized intraarticular injections. If symptoms do not improve or resolve within four to six weeks of treatment, escalation to include low-dose prednisone and possibly holding the ICI should be considered. If symptoms remain severe, consideration of holding the ICI should be given with use of high-dose prednisone (at least 1 mg/kg/day). Once symptom control is attained, steroids should be tapered slowly, over two to four weeks—if not longer.