More recently, in an effort to reconcile divergent opinions and minimize the risk of over- or undertreating, we proposed a risk stratification scheme with low-, intermediate-, and high-risk categories (see Figure 2, p. 43). According to this approach, patients at higher risk for ESRD should be treated more aggressively, whereas those at lower risk can be treated less aggressively.9,10 Failure to achieve a major response within the first four to six months should evoke consideration for intensifying therapy. Although based on data originating from various centers, this strategy has not been evaluated in the context of a trial.
We should focus our efforts on developing a more comprehensive strategy to guide the treatment of lupus. This strategy should both “fit the patient” and achieve timely and long-lasting remission with the lowest possible harm.
Mycophenolate Mofetil
Although, in some centers, the intense debate regarding the use of CY continued until the end of the millennium, a “new kid on the block” by the name of MMF appeared from the field of transplantation. Thus, at the beginning of this decade, several RCTs with short-to-medium–term (up to five years) follow-up claimed that MMF has at least comparable—if not superior—efficacy to CY in the treatment of proliferative LN. These studies generated a great deal of enthusiasm, passion, and anticipation in the community, with several investigators declaring the end of the CY era in national and international forums. Soon, a large multicenter, multiracial RCT (the Aspreva Lupus Management Study, or ALMS) was conducted, aiming to demonstrate superiority of MMF as induction and maintenance therapy.11 Patients with class III-V LN were initially randomized to receive open-label induction therapy with MMF (target dose 3 g/day) or IV-CY (0.5–1.0 g/m2 in monthly pulses) in combination with corticosteroids (tapered from a maximum dose of 60 mg/day) for 24 weeks. Patients achieving response or complete remission (primary outcome) were subsequently re-randomized to double-blind, placebo-controlled maintenance treatment with MMF or AZA, both plus corticosteroids.12 For the maintenance phase, the primary endpoint was time to treatment failure (defined as either flares, need to intensify therapy, doubling of serum creatinine, or death).
The ALMS-induction trial did not detect significantly different response rates between the two groups. Of interest, MMF responses were better in certain racial/ethnic groups such as black-race and mixed Latin American (Hispanic) patients. During the maintenance phase, the investigators found a failure rate of 32% in the AZA group versus 16% in the MMF group at three years. In contrast, Houssiau and colleagues reported that MMF and AZA were comparable as maintenance therapy for proliferative LN in a multicenter European trial.13 While awaiting the full report on ALMS-maintenance to sort out whether these results reflect differences in the outcomes used (single in the MAINTAIN trial, composite in ALMS), the number (n=105 in MAINTAIN, n=227 in ALMS) or ethnic background of the patients, the MAINTAIN study clearly demonstrates equivalence between AZA and MMF in European patients with moderately severe LN.14
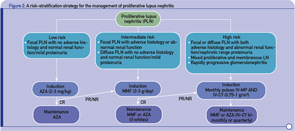
- Stratification of risk for progression to end-stage renal disease based on findings of longitudinal observational studies, and retrospective analyses of RCTs or clinical cohorts. Adverse histology denotes the presence of crescents and/or fibrinoid necrosis affecting >25% of glomeruli; glomerular sclerosis, tubular atrophy, or chronicity index >4; or chronicity index >3 and activity index >10. Impaired renal function denotes increase in serum creatinine or reduction in estimated glomerular filtration rate (calculated by the Cockcroft-Gault or the Modification of Diet in Renal Disease formula) by >25%, or proteinuria >4 g/24 hr.
- Renal response is assessed at six months. CR is defined as decrease in proteinuria to <1 g/day (or <0.3 g/day in LN diagnosed in the past six months) with normal serum albumin concentrations; inactive urine sediment; and improved or stable renal function. PR is defined as significant change in proteinuria (if nephrotic at baseline ≥50% decrease in proteinuria to <3 g/day; if non-nephrotic at baseline but not meeting the CR criteria) and improved or stable renal function.
Abbreviations: CR, complete response; PR, partial response; NR, no response.
