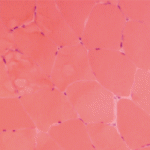
Similar issues are observed when studying the histopathology of these diseases. For example, the finding of lymphocytes in and around muscle fibers or in a perivascular location is consistent with the diagnosis of polymyositis or dermatomyositis. Yet, it is not uncommon to see nonspecific myopathic changes or even normal histology on muscle biopsy in some patients suspected of having an inflammatory myopathy.
According to Bohan and Peter, four criteria are needed to confirm definite disease, three to confirm probable disease, and two to confirm possible disease.1,2 Though this approach sounds simplistic, it can be difficult to apply this standard to individual patients. For example, is it appropriate to prescribe high-dose corticosteroids or other immunosuppressive therapies for a patient who fulfills just two criteria?
Bohan and Peter subdivided inflammatory myopathies into a number of subtypes including primary idiopathic polymyositis, primary idiopathic dermatomyositis, dermatomyositis (or polymyositis) associated with neoplasia, childhood dermatomyositis (or polymyositis) associated with vasculitis, and polymyositis or dermatomyositis associated with collagen-vascular disease (overlap group).1,2 Inclusion body myositis, first described in 1979, has subsequently been added as another subtype. Once again, this categorization is probably too simplistic.
Today, we believe that patients with classic forms of polymyositis, dermatomyositis, and inclusion body myositis have very characteristic changes in muscle histology. For example, polymyositis is characterized by infiltration of CD8+ lymphocytes invading muscle fibers with degenerating and regenerating fibers. In contrast, dermatomyositis has CD4+ T lymphocytes, B cells, and plasmacytoid dendritic cells in perivascular locations and perifascicular atrophy. Inclusion body myositis is associated with changes identical to polymyositis, but also demonstrates lined vacuoles and deposits of beta-amyloid and ubiquitin. These changes suggest a different underlying pathophysiology for each of the three conditions. However, the variations in pathology (variations that overlap or fail to show all the features described above) that are seen in so many patients suggest there are far more than just these three phenotypes. For example, one patient may have the pathology described for polymyositis, but also a rash characteristic of dermatomyositis. Another patient may show the reverse. Thus, it may be challenging to accurately diagnose some patients.
The identification of myositis-specific autoantibodies in many patients with inflammatory myopathies has increased our diagnostic abilities to some degree (see Table 2). Although a patient with the classic manifestation of the antisynthetase syndrome will have polymyositis or dermatomyositis plus interstitial lung disease, arthritis, fever, Raynaud’s phenomenon, and mechanic’s hands, there are other patients with circulating antisynthetase antibodies who have pulmonary disease without muscle involvement, or myositis without the other features. The presence of anti–signal recognition particle (SRP) antibodies usually carries a very poor prognosis, yet some patients with these antibodies respond well to therapy. Similarly, anti-Mi2 antibodies are generally, though not always, associated with a very good prognosis.