More than 10% of patients treated with statins will complain of muscle problems. Symptoms include myalgia, cramping, tenderness, or weakness often occurring in pectoral, quadriceps, biceps, abdominal, and low back muscles. CPK values range from normal to more than 10 times the upper limit of normal. Some patients develop an apparent immune-mediated necrotizing myopathy.5 This may be associated with circulating antibodies to HMG-CoA reductase and respond to vigorous immunosuppressive therapy.
Other drugs can also induce a polymyositis-like picture. Examples include penicillamine, interferon-α, and procainamide. Hydroxychloroquine, drugs that cause hypokalemia (diuretics and amphotericin), and licorice-induced hypokalemia can cause proximal muscle weakness. A common histopathologic feature for all these drugs is the presence of vacuoles seen on muscle biopsy.
Among the metabolic myopathies, myophosphorylase deficiency (McArdle disease), carnitine deficiency, and some mitochondrial myopathies can cause proximal muscle weakness, elevated CPK levels, and myopathic EMG changes. Muscle biopsies are not uniformly helpful since they may show nonspecific changes. The diagnosis may require sophisticated metabolic testing.
Evidence Regarding Therapy Is Lacking
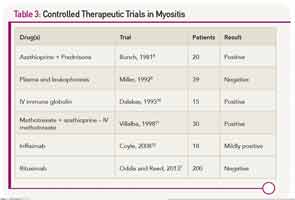
There are scant evidenced-based data to refer to when making therapeutic decisions for myositis (see Table 3). This may be one of the reasons why the prognosis for patients with polymyositis and dermatomyositis has not changed since the introduction of corticosteroids in 1949. About 20% to 40% of treated patients can enter a period of sustained remission, with the remaining 60% to 80% experiencing a continuous or fluctuating course.6 A concomitant malignancy, or the presence of circulating anti-SRP antibodies, suggest a poor prognosis. In most other situations, one cannot readily predict which patients will be good or poor responders.
Because idiopathic inflammatory myopathies are rare diseases, often have a very insidious onset (especially for inclusion body myositis), and can be challenging to diagnose, there have been few sizeable clinical trials. All but one of the published controlled trials had fewer than 50 patients. That study, the Rituximab in Myositis (RIM) trial, enrolled more than 200 patients from more than 30 centers. Despite the fact that more than 80% of subjects met the definition of improvement, the primary endpoints were not met.7
In the future, therapy may be guided by genetic or molecular testing. For example, rituximab may be more effective in patients with selected genetic signatures. Similarly, anti–interferon-α antibody (a biologic agent under development) may be effective in patients whose muscle has evidence for increased levels of interferon-α inducible genes. In the meantime, therapy for polymyositis and dermatomyositis remains empiric.