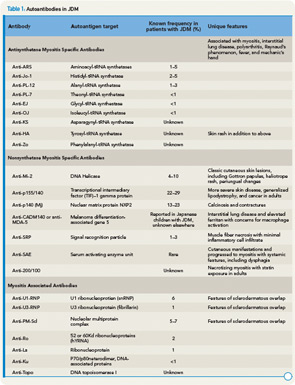
The presence of myositis-specific autoantibodies (MSAs) is rare in children. Anti-Jo-1 antibodies are the most prevalent, found in about 2% to 5% of JDM patients, and anti-Mi-2 antibody is seen in 5% (see Table 1).11 Antibodies targeting signal recognition particles (SRP) are seen in 2% to 3% of JDM. They share the same clinical presentation as adults with an acute onset of severe myositis of both proximal and distal muscles, myocarditis, and a resultant severe disability.
Recently, anti-p155/140 antibodies have been reported in 23% to 29% of JDM, and anti-p140 autoantibodies alone are seen in 20% to 30% of JDM and are associated with increased risk of calcinosis.12,13 Another 140kDa autoantibody, anti-MJ, is associated with severe disease with contractures and atrophy.14 While MSAs and MAAs are not always performed in children, they may be beneficial to help with prognosis and atypical clinical findings.
Recent Research Findings
It has long been appreciated that both humoral (autoantibodies and immune complexes) and cellular (T and B cells) components of the adaptive immune system contribute to the pathogenesis of JDM. However, in the last several years, there has been increasing evidence that the innate immune system, especially the upregulation of type I interferons and plasmacytoid dendritic cells may play an important role in the pathogenesis of disease.
Gene expression profiling of peripheral blood and affected tissues in JDM has revealed important insights into the molecular pathways underlying autoimmunity. The most prominent and consistent finding of these studies has been the presence of a gene signature characteristic of type I interferon (IFN) pathway activation, discovered first in DM muscle tissue and later identified in peripheral blood cells.15 The type I IFN pathway was further identified to include the cytokines/chemokines (MxA, IP-10, ITAC, MCP, interleukin 6) related to upregulation of this pathway.19
What do these studies tell us about disease mechanisms in JDM? Like many autoimmune diseases, there seems to be a genetic as well as environmental component. The genetic component appears to be multifactorial; HLA class genotype, especially HLA DQA1*0501 may help with persistence or development of maternal microchimerism.16 Cytokine polymorphisms may be involved as well. Whether there is an environmental trigger is unknown. Once the disease is initiated, there is maturation of dendritic cells leading to a type 1 interferon response and a cycle of inflammation develops with resulting damage to muscle and vascular endothelium, activation of B cells and CD4+ T cells, and overexpression of MHC class 1 on cell surfaces. It is believed that once this cycle begins, it may be halted by immunosuppressive therapy.
Therapy and Future Directions
The mortality rate of JDM was markedly reduced following the inception of corticosteroid therapy. With the use of methotrexate, the mean time of corticosteroid use fell to 10 months, compared with a historical mean time of 27 months.17 Surveys of North American pediatric rheumatologists indicate that most practitioners will treat typical JDM with corticosteroids and methotrexate, although there is great discrepancy between dosing regimens.18 Medications for refractory disease vary, but include hydroxychloroquine, intravenous immunoglobulin, rituximab, cyclophosphamide, cyclosporine A, azathioprine, tacrolimus, and mycophenolate mofetil.