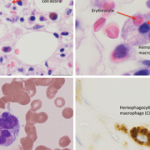
NEW YORK (Reuters Health)—Immunosuppressive therapy with infliximab (Remicade) for inflammatory bowel disease (IBD) in pediatric patients is not associated with increased risk of malignancy or hemophagocytic lymphohistiocytosis (HLH), according to a Janssen study.
Dr. Jeffrey S. Hyams, who worked on the study, calls the finding “reassuring.” He adds in an email to Reuters Health, “our data do suggest an association between thiopurine therapy not only with malignancy, but with another serious condition (HLH) as well.”
In a paper published online on Feb. 10 in Gastroenterology, Dr. Hyams of Connecticut Children’s Medical Center in Hartford and colleagues note that associations between IBD and intestinal malignancies are well established.
Prior reports on the risk of malignancy and HLH associated with therapy for pediatric patients, they write, “have been limited to largely retrospective studies with small sample sizes.”
To investigate further, the team collected and analyzed data from 2007 to 2014 on more than 5,700 patients no older than 17 years at enrollment. They were taking part in a prospective study involving outcomes of Crohn’s disease, ulcerative colitis or unclassified IBD.
Nearly half received one or more biologics, including infliximab (94.6%), adalimumab (34.0%) and certolizumab (5.1%). In addition to the anti-TNF alpha therapy, 232 of these patients also received other biologics.
There were 15 cases of malignancy over the course of the study, and all five of the patients who developed HLH had been exposed to thiopurine. Four patients were exposed to thiopurines in the absence of biologics and all five cases of HLH occurred during active thiopurine therapy.
Ten patients were exposed to infliximab; of these, five were also exposed to adalimumab, six were also exposed to methotrexate and nine were also exposed to thiopurines.
Thus, say the researchers, “13 of the 15 patients with malignancies were exposed to thiopurines; and the majority of these patients developed malignancy after less than 5 years of thiopurine exposure.”
Calculated standardized incidence ratios over more than 24,000 patient-years of follow up showed no increased risk of malignancy or HLH in patients exposed to infliximab as the only biologic compared to those not exposed to biologics. This was the case even when patients were stratified by thiopurine exposure.
“Though these very serious complications remain rare,” concludes Dr. Hyams, “clinicians may want to re-evaluate alternatives to azathioprine use in their patients.”
Janssen Scientific Affairs LLC funded and was involved in all aspects of the study, including manuscript preparation. Dr. Hyams has served as a consultant to Janssen OrthoBiotech and three of his coauthors are or were employees of Janssen at the time of the study. A number of other authors also reported relationships with the company.
Reference
- Hyams JS, Dubinsky MC, Baldassano RN, et al. Infliximab not associated with increased risk of malignancy or hemophagocytic lymphohistiocytosis in pediatric patients with inflammatory bowel disease. Gastroenterology. 2017 Feb 10. pii: S0016-5085(17)30148-8. doi: 10.1053/j.gastro.2017.02.004. [Epub ahead of print]