The role of B cells in autoimmune inflammatory diseases is emerging as one of the most interesting, if not controversial, topics in the development of targeted therapies. This has been highlighted recently by the announcement of a second positive trial of a B cell–targeted treatment in systemic lupus erythematosus (SLE) and by another report of progressive multifocal leukoencephalopathy (PML) in a rheumatoid arthritis (RA) patient treated with rituximab. The diseases considered for B cell–targeted therapy run the spectrum from traditionally viewed B cell–centric diseases such as SLE, to RA, to conventionally viewed T cell–mediated conditions such as multiple sclerosis (MS) (see Table 1, at right). Correspondingly, there are an increasing number of B cell–directed therapies in development for the treatment of autoimmune disease.
B-cell depletion therapy is now routinely used in the treatment of diverse diseases, especially those refractory to conventional immunosuppressive therapy. Moreover, what once was viewed as a one-time therapy often is now used recurrently. It is critical to understand the impact of B-cell depletion on normal immune responses, the reasons B cell–targeted therapies may be efficacious in such a broad range of diseases, potential differences in B cell–directed therapies in efficacy and mechanism of action, and determination as to whether there are subsets of patients who will respond particularly well to B-cell approaches as opposed to other treatment modalities.
The Multi-layered Role of B Cells in Autoimmune Disease
To understand the efficacy of B-cell therapies, as well as treatment response heterogeneity, we need to consider the role of the B cell in autoimmune diseases. As a starting point, to borrow a phrase, autoimmune diseases are not created equal. Certain diseases may be predominantly autoantibody mediated, others may involve B cells via autoantibody-independent mechanisms such as cytokine production or antigen presentation, other disease may be T-cell driven, and many undoubtedly represent some combination of these.
The importance of antibody-independent roles for B cells is often underappreciated, but is highlighted by certain important findings: 1) In mouse lupus, B cells are critical to the development of disease even when they are unable to secrete autoantibodies1; 2) In at least some human autoimmune diseases, the efficacy of B-cell depletion is dissociated from changes in levels of autoantibodies; and 3) The most compelling efficacy data for B-cell depletion occurs in diseases that are traditionally viewed as T-cell driven (e.g., RA and MS), suggesting a role for B cells in regulating other immune cells.
B cells may participate in immune dysregulation at multiple levels by presenting antigens and providing costimulatory signals to T cells and helping to regulate and organize inflammatory responses through cytokine and chemokine secretion (such as interleukin-10, interleukin-6, interferon-γ, and lymphotoxin-a [LTa]).2–4 A particularly important function of B cells may be the direct effect they have on lymphoid neogenesis through the production of LTa. The formation of tertiary or ectopic lymphoid tissue is a process that may lead to dysregulated B/T-cell interactions and local amplification of autoimmune responses in multiple autoimmune diseases, including RA, Sjögren’s syndrome, type I diabetes, MS, inflammatory bowel disease, Hashimoto’s thyroiditis, and SLE.5
For diseases or subsets of disease where autoantibodies play a more central pathogenic role (e.g., autoimmune hemolytic anemia in SLE or neuromyelitis optica), it is likely important to truce the production autoantibodies with therapy. For B cell–depletion therapy with anti-CD20, this reduction may occur indirectly if the autoreactive plasma cells are short lived and precursor B cells are efficiently depleted. Targeting long-lived plasma cells is more challenging, because these cells appear to be resistant to most therapies, including anti-CD20.
B Cell–Targeted Therapy in RA and SLE
A number of different approaches to targeting B cells have been utilized: 1) B-cell depletion with monoclonal antibodies against B cell–specific molecules (e.g., anti-CD20); 2) induction of negative signaling in B cells (e.g., anti-CD22); and 3) blocking B-cell survival and activation factors (e.g., anti-BAFF). (See Table 2, p. 26.)
TABLE 1: The Expanding Spectrum of Diseases Treated with B Cell–Depletion Therapy
FDA approved
- RA (anti-TNF failures)
Randomized controlled trials completed
- SLE general
- SLE nephritis
- Relapsing-remitting MS
- Primary progressive MS
- Antineutrophil cytoplasmic antibody (ANCA)–mediated vasculitis
- Type 1 diabetes
Others
- Neuromyelitis optica
- Sjögren’s
- Scleroderma
- Myositis
- Antiphospholipid syndrome
- Idiopathic thrombocytopenic purpura (ITP)
- Thrombotic thrombocytopenic purpura (TTP)
- Transplant rejection
- Inflammatory bowel disease
- Chronic graft-versus-host disease
- Blistering skin diseases
- Idiopathic membranous nephropathy
- Pulmonary hypertension
- Hepatitis C cryoglobulinemia
- IgM-associated polyneuropathy
- Uveitis
- Autoimmune paraneoplastic syndromes
B Cell–Depletion Therapy
Since CD20 is expressed on immature, naive and memory B cells but not mature plasma cells, in the use of rituximab (anti-CD20) in patients with lymphoma and RA, B cells are depleted, but serum IgG does not change significantly. The success of several large phase II–III trials of rituximab in RA led to its approval for tumor necrosis factor (TNF) refractory disease in 2006. As rheumatologists have become more familiar with using this therapy in RA, there has been expanding off-label use in other rheumatic diseases, including SLE and antineutrophil cytoplasmic antibody vasculitis.
Despite considerable enthusiasm for rituximab in SLE, two recent placebo-controlled trials in nonrenal lupus (EXPLORER) and renal lupus (LUNAR) failed to meet primary endpoints. Although EXPLORER is clearly the best trial we have so far on the use of rituximab for SLE, it does have problems. It was designed to enter patients who had very active disease and therefore were treated aggressively with moderate- to high-dose glucocorticoids. This approach makes the detection of a benefit difficult over the short term. Interestingly, the rituximab-treated group did have a serological response by the one-year endpoint, suggesting that some clinical benefit might have accrued with longer follow-up.
Other problems with the EXPLORER trial include the outcome measures used and the heterogeneous nature of SLE itself. Because different manifestations of SLE may result from different mechanisms, one targeted therapy is unlikely to be effective for all patients. Finally, it is also worth noting that the most impressive open-label results with rituximab for SLE have been observed in refractory patients treated with one or two low doses of cyclophosphamide in combination with rituximab, an approach not used in either EXPLORER or LUNAR.6
Other Anti-CD20–Directed Therapies
Other monoclonal antibodies that target CD20 are in various phases of development, including ocrelizumab (humanized anti-CD20). Theoretically, a fully human antibody may be better tolerated during infusions due to less immunogenicity. This situation could translate into more complete B-cell depletion, especially in SLE where human antichimeric antibodies are more common after rituximab, which has mouse immunoglobulin sequences. It seems logical that our goal should be to achieve as complete a B-cell depletion as possible, although this may not equate to greater efficacy in all cases.
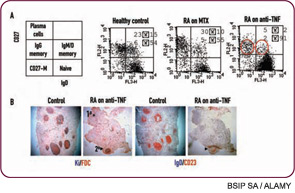
Although anti-CD20 is usually effective in depleting B cells from peripheral blood, success in depleting B cells from other sites, such as lymph nodes or tertiary lymphoid tissues, may be highly variable. Failure to deplete in these tissue sites may lead to nonresponse or early relapse. Combination therapy, either with conventional disease modifying agents or other biologics such as cytokine blockade, may achieve better depletion. Along these lines, we have found that treatment of RA with etanercept has unexpected effects on the B-cell compartment with decreases in memory B cells and altered lymphoid architecture (see Figure 1, p. 25).7 These findings suggest a convergence of mechanism between TNF blockade and B-cell depletion therapy and raise the interesting speculation regarding potential synergy. Along these lines, it is interesting that there are anecdotal reports of improved efficacy of TNF blockade after B cell–depletion therapy (in patients with a prior inadequate response to anti-TNF). Interestingly, the BELONG trial, a phase III trial of ocrelizumab for lupus nephritis that has been underway, includes an arm with combination anti-CD20/cyclophosphamide that may also act synergistically for more effective B-cell depletion. This trial may help define whether cyclophosphamide should be used with anti-CD20 in the treatment of SLE.
Other B Cell–Targeted Therapies
Epratuzumab is a humanized anti-CD22 that also induces B-cell depletion, although the depletion is less pronounced than with anti-CD20 and is preferentially of naive and transitional B cells. However, anti-CD22 also blocks the activation and proliferation of B cells, possibly acting as a negative regulator of B-cell function. A recent Immunomedics press release regarding a phase IIb trial of epratuzumab in SLE reported superior response rates compared with placebo at Week 12.
Inhibition of B-cell activation and survival is another strategy for targeting the B-cell compartment. This approach could include blockade of costimulatory interactions that promote B-cell activation (e.g., anti-CD40L or CTLA4-Ig) or neutralization of B-cell survival factors/cytokines, such as B cell–activating factor belonging to the TNF family (BAFF). Belimumab is a fully human monoclonal antibody against BAFF. After initial encouraging results in a phase II trial in SLE, two phase III trials were initiated, have recently completed recruitment, and preliminary press releases from Human Genome Sciences and GlaxoSmithKline have reported favorable clinical responses compared with placebo (based on a composite SLE responder index). Given these initial reports, we should consider whether BAFF blockade would be expected to yield different results compared with anti-CD20. Indeed, this would be surprising given that both agents deplete B cells, with anti-CD20 causing even more pronounced B-cell depletion and likely better depletion of memory B-cell subsets. On the other hand, BAFF blockade may have additional effects on other immune cells important in SLE, including T cells and dendritic cells. Moreover, rituximab causes a compensatory increase in BAFF, which may theoretically have adverse effects on B-cell selection. Nevertheless, I suspect that the distinct trial results with BAFF blockade versus anti- CD20 in SLE are more related to trial design than biologic differences between the two therapies.
Another potential explanation for the disappointing results of B-cell depletion in SLE is the lack of effect on autoantibodies and long-lived plasma cells. Atacicept is a receptor fusion protein that blocks both BAFF and its related cytokine APRIL and may be unique in its dramatic effects on plasma cells, with reductions in total immunoglobulin (Ig) G, IgM, and autoantibodies in phase I studies in both RA and SLE.8 Because of its effects on plasma cells, transmembrane activator and calcium-modulating and cyclophilin ligand interactor (TACI)-Ig would appear to have great potential in autoantibody-mediated diseases. However, the challenge will be to eliminate autoantibody-producing plasma cells without increasing the risk of infection due to adverse effects on protective antibodies.
TABLE 2: Approaches to Target B Cells
B cell depletion
- Rituximab (anti-CD20)
- Ocrelizumab (humanized anti-CD20) (Phase III in RA and SLE)
- Anti-CD20 (small modular immunopharmaceutical [SIMP]) (Phase III in RA)
- Ofatumumab (anti-CD20 from humanized mice) (Phase III in RA)
Inhibitory signaling
- Epratuzumab (anti-CD22) (Phase II in SLE)
Cytokine blockade
- Belimumab (anti-BAFF) (advanced development in RA, SLE)
- BR3-Fc (Phase I RA)
- Anti-BR3 (Phase I RA)
- Atacicept (TACI-Ig) (Phase I, II complete in RA and SLE)
Clinical Utilization and Safety
Given that B-cell depletion is irreversible until reconstitution occurs (and persists for long periods during which the patient may not make antibodies to infections or immunization), clinicians may be less comfortable using this therapeutic approach in RA instead of a TNF blockade, which can be stopped. However, a recent meta-analysis supports prior literature regarding the safety of B-cell depletion in that treatment of RA with rituximab was not associated with an increased incidence of serious infections, even after repeated courses, or with TNF blockade after B-cell depletion.9-11 The caveat here is that the database is relatively small for RA and even smaller for SLE. Moreover, there have been reports of fulminant hepatitis B reactivation and rare cases of PML. Thus, patients should be screened for hepatitis B prior to the use of rituximab, and viral prophylaxis should be considered in high-risk patients.
Unfortunately, there are no screening methods to identify patients at risk for development of PML. A third case of PML was recently reported in an RA patient receiving rituximab, notably without prior anti-TNF treatment or risk factors for PML development, such as malignancy or heavy immunosuppression. Although the frequency of PML in RA patients receiving rituximab is rare (3 in 100,000 patients), a recent press release from Genentech notes that the information to date suggests that patients with RA who receive rituximab have an increased risk of PML. Current U.S. Food and Drug Administration recommendations are that rituximab only be used in later-stage RA patients who have inadequately responded to anti-TNF therapy, which has been our practice to date. Given that primary and secondary immune responses may be compromised even a year after therapy, it is also recommended that immunizations be given one month prior to starting rituximab, and the yearly influenza vaccine given as late as possible after the last dose.12 This would include vaccination for the novel H1N1 virus, but avoidance of live vaccines, similar to the practice for other immunocompromised populations. I also routinely monitor serum immunoglobulin levels (yearly) given the occurrence, albeit rare, of hypogammaglobulinemia and the fact that replacement intravenous immunoglobulin can be considered in the setting of severe hypogammaglobulinemia (serum IgG<200-300 mg/dl) (see Table 3, above).
TABLE 3: Indications and Recommendations for B Cell–Depletion Therapy
Use
- FDA approved for the treatment of RA patients refractory or unable to use anti-TNF
- Other autoimmune diseases when conventional therapy fails or has unacceptable toxicity: SLE, MS, ANCA-vasculitis, ITP, etc.
Screening prior to use of rituximab
- History of severe, unusual, or frequent infections
- History of high risk for HIV or tuberculosis
- Serology for hepatitis B (HBsAg, anti-HBs, anti-HBc)
- Serology for varicella-zoster virus (VZV) for patients who have not had chickenpox or been immunized should be considered in patients who are heavily immunosuppressed
- IgM, IgG, IgA levels (and yearly with ongoing treatment)
Immunization
- Avoid live virus vaccines
- If possible, complete immunizations one month prior to starting rituximab
- After rituximab, continue to administer recommended vaccines, including yearly influenza vaccine, but try to give as late as possible after the last rituximab dose
Prophylaxis
- Influenza: Consider antiviral chemotherapy during outbreak in selected patients
- Hepatitis B: Patients at high risk for reactivation, consider prophylaxis with lamovudine
- VZV: Post-exposure prophylaxis should be considered in seronegative patients who have additional risk factors
Research Areas
- Better understanding of dosing and frequency of treatment
- Consider monitoring of B-cell counts on a case-by-case basis
- Use for induction versus maintenance with chronic B-cell depletion
Another question that frequently arises is whether it is necessary to monitor B-cell counts. In large series of RA patients, there was no clear temporal relationship between peripheral B-cell return and loss of response, making such a recommendation difficult to substantiate. On the other hand, the detection of residual B cells using high sensitivity flow and the return of B cells increases the risk of relapse. In SLE, where incomplete B-cell depletion is more common, I routinely assess for depletion (one to two months after therapy) and reconstitution (at least at disease flare or if repeat treatment is being considered). Is long-term depletion of B cells necessary to maintain clinical benefit? In RA, transient B-cell depletion can ameliorate disease for a prolonged period, but typically not indefinitely. Repeated courses of B-cell depletion can prevent disease relapse.10 However, the optimal timing of repeated B-cell depletion remains unclear. Given the heterogeneity in treatment responses, my approach is to tailor the treatment to the patient and consider repeat B-cell depletion when disease begins to relapse. Biomarkers to assess the need for re-treatment and even to stratify patients into those likely to respond to B-cell depletion in the first place are sorely needed. Recent reports do suggest that seropositive RA patients tend to have better responses to B-cell depletion.
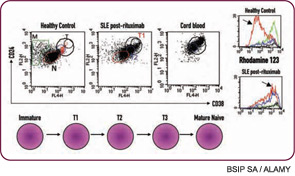
Another underappreciated point is that the therapeutic effect of B cell–depletion therapy likely does not lie solely in the depletion of B cells. Not all of the effects of B cells promote autoimmunity. In particular, my colleagues and I have observed that the nature of B-cell reconstitution affects responses in SLE, with long-term responders demonstrating a prolonged expansion of immature transitional B cells after reconstitution (see Figure 2, above left).13 Of significant interest, expansion of transitional B cells also correlates with prevention of diabetes after B-cell depletion in nonobese diabetic mice.14 Thus, we postulate that the therapeutic benefit of B-cell depletion depends on the balance of pathogenic and protective B-cell functions. If this is true, long-term B-cell depletion may not be necessary or advisable, given that regulatory B cells are not distinguished from pathogenic B cells with current approaches.
Conclusions and Future Directions
Targeting B cells appears to be effective even in diseases where tissue damage is mediated by T cells, probably because B cells function as antigen-presenting cells as well as antibody-producing effector cells. B-cell depletion therapy can be remarkably efficacious in RA and remains a treatment strategy in refractory SLE. Recent controlled clinical trials of B cell targeting agents in SLE have had variable benefit but have contributed to our understanding of how to do trials in lupus and have recently shown promise. New agents capable of affecting long-lived plasma cells are now being developed. These agents should allow eradication of autoantibodies but will need to be used carefully to prevent infectious complication and to ensure that autoimmune plasma cells do not repopulate the long-lived plasma cell compartment. Important areas of future study include the identification of biomarkers, such as unique B-cell subsets and autoantibodies profiles, to better define responsive patient groups, determine the optimal timing of treatment and role of combination therapy, and the place of B-cell depletion in the therapeutic hierarchy.
Acknowledgments: Dr. Anolik has been supported by several grants including U19 Autoimmunity Center of Excellence AI56390, R01 AI077674-01A1, and the Lupus Research Institute. The contributions of collaborators, including Dr. Sanz and Dr. Looney, are especially noted.
Editor’s note: Dr. Anolik has served as a consultant for Genentech and Roche and has received grants for preclinical and translational studies from Proteolix and Amgen.
Dr. Anolik is associate professor of medicine in the division of allergy, immunology, and rheumatology of the department of medicine at the University of Rochester in New York.
References
- Shlomchik MJ, Madaio MP, Ni D, Trounstein M, Huszar D. The role of B cells in lpr/lpr-induced autoimmunity. J Exp Med. 1994;180: 1295-1306.
- Mamula MJ, Fatenejad S, Craft J. B cells process and present lupus autoantigens that initiate autoimmune T cell responses. J Immunol. 1994;152:1453-1461.
- Linton PJ, Harbertson J, Bradley LM. A critical role for B cells in the development of memory CD4 cells. J Immunol. 2000; 165:5558-5565.
- Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189:1639-1648.
- Lorenz RG, Chaplin DD, McDonald KG, McDonough JS, Newberry RD. Isolated lymphoid follicle formation is inducible and dependent upon lymphotoxin-sufficient B lymphocytes, lymphotoxin beta receptor, and TNF receptor I function. J Immunol. 2003;170:5475-5482.
- Lu TY, Ng KP, Cambridge G, et al. A retrospective seven-year analysis of the use of B cell depletion therapy in systemic lupus erythematosus at University College London Hospital: the first fifty patients. Arthritis Rheum. 2009;61:482-487.
- Anolik JH, Ravikumar R, Barnard J, et al. Cutting edge: anti-tumor necrosis factor therapy in rheumatoid arthritis inhibits memory B lymphocytes via effects on lymphoid germinal centers and follicular dendritic cell networks. J Immunol. 2008; 180:688-692.
- Munafo A, Priestley A, Nestorov I, Visich J, Rogge M. Safety, pharmacokinetics and pharmacodynamics of atacicept in healthy volunteers. Eur J Clin Pharmacol. 2007;63:647-656.
- Salliot C, Dougados M, Gossec L. Risk of serious infections during rituximab, abatacept and anakinra treatments for rheumatoid arthritis: meta-analyses of randomised placebo-controlled trials. Ann Rheum Dis. 2009;68:25-32.
- Keystone E, Fleischmann R, Emery P, et al. Safety and efficacy of additional courses of rituximab in patients with active rheumatoid arthritis: an open-label extension analysis. Arthritis Rheum. 2007;56:3896-3908.
- Genovese MC, Breedveld FC, Emery P, et al. Safety of biologic therapies following rituximab treatment in rheumatoid arthritis patients. Ann Rheum Dis. 2009;68;1894-1897.
- Looney RJ, Srinivasan R, Calabrese LH. The effects of rituximab on immunocompetency in patients with autoimmune disease. Arthritis Rheum. 2008;58:5-14.
- Anolik JH, Barnard J, Owen T, et al. Delayed memory B cell recovery in peripheral blood and lymphoid tissue in systemic lupus erythematosus after B cell depletion therapy. Arthritis Rheum. 2007;56:3044-3056.
- Hu CY, Rodriguez-Pinto D, Du W, et al. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest. 2007;117:3857-3867.