This is Part Two of a two-part series. Part One, on SLE and connective tissue disease, appeared in the February issue of The Rheumatologist (p. 1).
Laboratory testing is an essential element in the diagnosis and management of patients with rheumatic disease. This series focuses on a diverse array of serological markers that can provide unique information on the status of the patient’s immune system that is important in clinical evaluation as well as scientific inquiry. These tests help in the diagnosis of a particular disease and, importantly, they may help monitor disease activity. Indeed, immunological testing represents one of the bedrocks of rheumatology and is a distinguishing feature of our specialty.
While there are many available tests, the approach to serology follows the traditional approach of any laboratory study. Critical in the interpretation of any serological test is determining its sensitivity (i.e., the proportion of patients with the target disorder who have a positive test), specificity (i.e., that proportion of patients who are free of the target disorder and have negative or normal test results), and positive and negative predictive values. The predictive values calculate the likelihood that disease is present or absent based on test results using the test’s sensitivity, specificity, and the probability of disease before the test is performed (pretest probability). This review will cover tests for rheumatoid arthritis (RA).
Rheumatoid Factors
Rheumatoid factors (RFs) are antibodies directed against the Fc portion of immunoglobulin G (IgG). The RF, as currently measured in clinical practice, is an IgM RF, although other immunoglobulin types, including IgG and IgA, have been described.1,2 ELISA or nephelometry generally detects the presence of RF; latex agglutination (a test fraught with technical problems) is still sometimes used. Testing for RF is primarily used for the diagnosis of RA; however, RF may also be present in other rheumatic diseases and chronic infections.3
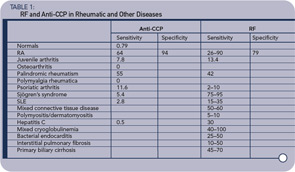
Patients may have detectable serum RF in a variety of rheumatic disorders, many of which share similar features, such as symmetric polyarthritis and constitutional symptoms. These include idiopathic juvenile idiopathic arthritis, palindromic rheumatism, and Sjögren’s syndrome (see Table 1, p. 19).1,2
Nonrheumatic disorders characterized by chronic antigenic stimulation (especially with circulating immune complexes or polyclonal B lymphocyte activation) commonly induce RF production (see Table 1, below right). Included in this group are indolent or chronic infection, as with subacute bacterial endocarditis or hepatitis B or C virus infection, and inflammatory or fibrosing pulmonary disorders, such as sarcoidosis, malignancy, and primary biliary cirrhosis. Rheumatoid factors have been found in up to 5% of young, healthy individuals. The reported incidence may be higher in elderly subjects without rheumatic disease, ranging from 3% to 25%.
The higher the titer, the greater the likelihood that the patient has a rheumatic disease. There are, however, frequent exceptions to this rule, particularly among patients with one of the chronic inflammatory disorders noted above. Furthermore, the use of a higher titer for diagnosis decreases the sensitivity of the test at the same time as it increases the specificity. RF-positive patients with RA may experience more aggressive and erosive joint disease and extra-articular manifestations than those who are RF negative. Similar findings have been observed in juvenile RA. These general observations, however, are of limited utility in an individual patient because of wide interpatient variability. In this setting, accurate prediction of the disease course is not possible from the RF alone. RF titer rarely correlates with the degree of clinical activity.
Antibodies to Citrullinated Proteins
There has been considerable interest in developing a better test for the diagnosis of RA, which has greater sensitivity and specificity than the tests that detect RFs. Within the last decade, as an outgrowth of determining the molecular specificity of antifilaggrin, antikeratin, and antiperinuclear antibodies, it was recognized that many patients with RA have antibodies to citrullinated proteins. Proteins that are citrullinated have had an arginine replaced by citrulline, a minor amino acid. This posttranslational modification is mediated by the enzyme peptidyl arginine deiminase 4 (PAD4).
PAD4 is the form of PAD associated with RA. It has been found in bloodstream granulocytes and is localized in the cell nucleus. Its only known activator is a component in cigarette smoke, but its activation is also associated with inflammation. Much more work needs to be done to determine what specifically in RA activates (and downregulates) PAD. Because increased activity of PAD4 has been demonstrated in synovial fluid and synovium of patients with RA and other inflammatory arthritides, but generally not OA, it appears to be associated with inflammation. Citrullination of proteins has also been in noted in multiple sclerosis. The primary physiologic role for PAD enzymes (and the conversion of arginine to citrulline) appears to be related to regulation of nucleosome, that is, histone function. However, the development of antibodies to citrullinated proteins is relatively specific to RA.
To facilitate analysis of antibodies to citrullinated proteins, a number of peptide antigens containing citrulline were created, although these have had a variable degree of sensitivity for recognizing patients with RA. However, a cyclic peptide containing citrulline was found to have a high sensitivity and was subsequently utilized to develop a commercial assay. This test, named anti-CCP (for cyclic-citrullinated peptide) has now been studied extensively, especially in Europe, and it has better sensitivity and specificity for the diagnosis of RA than tests that detect RF. A number of commercial firms have developed different assays for the detection of these antibodies to CCP, and the assays have been called CCP1, CCP2, CCP3, etc.
There have been numerous claims regarding the superiority of one assay compared with another, and this requires further investigation. In addition, a number of groups have assayed antibodies to other citrullinated proteins—especially fibrinogen and vimentin—and compared these assays to anti-CCP, with variable results. These latter assays are not, at present, commercially available. Most studies to date on examining the frequency, sensitivity, specificity, predictive value of anti-CCP in RA relate to CCP2—which is the assay I employ in my laboratory.
Since the original description of anti-CCP antibodies,4,5 multiple studies have established the clinical utility of measuring these anticitrulline autoantibodies via commercial assays (anti-CCP) as a biomarker in RA.6 In addition to displaying a pooled sensitivity of 67% and specificity of 95% for the diagnosis of RA,7 it is now established that anti-CCP autoreactivity is prognostic for disease severity, radiographic erosions, and the early recognition of RA.6,8 Rather than supplanting measurement of RF, the utility of anti-CCP has been demonstrated both by itself and in combination with measurement of serum RF. The consensus that has emerged from these studies is that, while anti-CCP and RF are frequently overlapping in their presence and prognostic significance, they are also independently informative in a significant subset of patients. The differences between subsets of RA patients with distinct autoantibody profiles remains poorly understood.
In addition to performance of these antibodies as a biomarker in the clinical setting, recent evidence suggests a pathogenic association between anti-citrulline autoantibodies (measured by the anti-CCP test) and RA.9 Retrospective studies in multiple cohorts have demonstrated that measurable levels of anti-CCP autoantibodies frequently precede development of clinical arthritis with evidence for an increase in frequency and titers proximal to disease onset.10,11 At a population level, recent studies have shown that the presence of anti-CCP correlates with the presence of specific HLA-DR alleles with a particularly strong association with the “shared epitope” genes.12-15 At the level of basic mechanisms, citrullinated peptides from known autoantigens bind more avidly to MHC shared epitope than native peptides and are thereby capable of activating CD4+ T-lymphocytes. This property provides a plausible molecular basis for the genetic association seen in RA patients.16
An additional connection between smoking—a putative environmental trigger for RA—and anticitrulline autoantibodies provides further potential insight into mechanisms of disease evolution. It has long been appreciated that smoking confers an increased risk of RA.17-20 Although this finding has not been replicated in all cohorts, recent studies demonstrate that the increased risk conferred by tobacco exposure is present in the anti-CCP+ subset of subjects,6 and is associated with HLA-DR alleles.14,15 Furthermore, pulmonary lavage samples from smokers show citrullination of cellular proteins that is not present in nonsmoking subjects, providing a potential source of citrullinated antigens to induce autoantibody production. Moreover, citrulline posttranslational modification of tissue proteins occurs in diseased joints, and joint tissue citrullination levels are markedly elevated in the context of inflammation in both humans and experimental rodents.21-25
Evidence from animal models further supports the potential contribution of anticitrulline antibodies to the pathogenesis of inflammatory arthritis. Recent studies in the collagen induced arthritis model have demonstrated that anticitrulline autoantibodies are induced by immunization with collagen and that establishing tolerance to citrullinated peptides blocks development of disease.23 Perhaps more compelling evidence for the pathogenic role of these antibodies is the ability to passively transfer anticitrulline antibodies to mice with subclinical arthritis and initiate robust symmetric inflammatory synovitis. Interestingly, passive transfer of anticitrulline antibodies into mice without any inflammatory stimulus does not elicit arthritis, and citrullination of joint tissue proteins is evident in mice with subclinical collagen-induced arthritis (CIA).
Taken together, these observations suggest a new disease model where the combination of anticitrulline antibodies and inflammation-induced citrullination of tissue proteins can conspire to induce an autoimmune inflammatory arthritis.9 The clinical utility of testing for anti-CCP versus RF is summarized in Table 1 (below). The anti-CCP test, while having similar sensitivity for the presence of RA, has much greater specificity. In addition, antibodies to CCP are rarely found in patients with other rheumatic conditions and infectious diseases where RF is more frequently found. Anti-CCP is even found frequently before the diagnosis of RA. These observations suggest that the anti-CCP test may be more useful for the diagnosis of RA than are RF tests—or that it at least should be part of the diagnostic algorithm.
Recently, my colleagues and I have examined a large cohort of patients with early arthritis who were followed for at least six months.26 The substitution of anti-CCP for the ACR classification criteria subcutaneous nodules and erosions improved on the ACR classification criteria for the earlier identification and classification of those patients who eventually developed RA. On the basis of these findings, we recommend that, if one suspects that a patient has RA, one should test for both RF and anti-CCP.
What do we do with these results in our Arthritis Center? We have observed a gradual trend for earlier and more aggressive treatment with disease-modifying antirheumatic drugs (DMARDs) in the patient who presents with a symmetric polyarthritis, and sometimes even a monoarthritis, who has a positive anti-CCP test. This approach is in marked contrast with the way the RF test was used, due to the lower specificity of this test in early arthritis in predicting RA. However, we rarely add a biological (e.g., TNF blocker) to the treatment regimen unless the patient has failed two DMARDs, clearly has inflammation, and is felt clinically to probably have RA. However, more carefully constructed clinical trials are needed to guide us on when and how to treat the early arthritis patient, because not all of them develop RA.
Summary
What does the future hold? First, in respect to antinuclear antibodies (ANAs, covered in Part One of this series), I predict that testing will become more automated, based on both economic pressures and improvement in solid-phase immunoassays. I suspect this will be coupled with the recognition that systemic lupus erythematosus, scleroderma, and Sjögren’s each represent a spectrum of related disorders. I predict that the combination of good clinical description, genetic markers, and assays for biomarkers via proteomics, will better divide up related subsets, resulting in better testing as well as therapy.
With respect to RA, I predict less reliance on the misnamed rheumatoid factor test, better recognition that RA is also probably a spectrum of related diseases (not just seropositive and seronegative RA) and that, likewise, better serological, and genetic testing will help define patient subsets. I strongly believe that different subsets of “RA” patients will have antibodies to different citrullinated proteins, and that we will end up having a RA-citrullinated panel of tests. Hopefully, this subsetting with coupling of better therapies, probably guided by genomics, will improve treatment.
Progress in rheumatology serology has been slow through the 1940s (RF and LE cell prep), 1950s (ANA and anti-DNA), 1960s (Sm, RNP, Ro, La), and 1990s (CCP). However, the understanding of the genetics of the disorders discussed here—as well as the therapies for them—has been accelerating. Let us hope that the future will bring new and better discoveries.
Acknowledgement: I am indebted to the work of many authors of UpToDate in Medicine, whose work provided a useful framework for the development of this paper as well as to Drs. Robert Shmerling, David Lee, and Donald Bloch with whom I have written papers/chapters on this same subject.
Dr. Schur is professor of medicine at Harvard Medical School and the division of rheumatology, immunology and allergy in the department of medicine at Brigham and Women’s Hospital in Boston.
References
- Shmerling RH. Origin and utility of measurement of rheumatoid factors. UpToDate. October 2008.
- Shmerling RH, Delbanco TL. The rheumatoid factor: An analysis of clinical utility. Am J Med. 1991;91:528.
- Kavanaugh AF, Solomon DH, the American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines. Guidelines for immunologic laboratory testing in the rheumatic diseases. Anti-DNA antibody tests. Arthritis Rheum. 2002;47:546-555.
- Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998;101:273-281.
- Girbal-Neuhauser E, Durieux JJ, Arnaud M, et al. The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro)filaggrin by deimination of arginine residues. J Immunol. 1999;162:585-594.
- Lee DM, Phillips R, Hagan EM, Chibnik LB, Costenbader KH, Schur PH. Quantifying anti-cyclic citrullinated peptide titres: Clinical utility and association with tobacco exposure in patients with rheumatoid arthritis. Ann Rheum Dis. 2009;68:201-208.
- Nishimura K, Sugiyama D, Kogata Y, et al. Meta-analysis: Diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med. 2007;146:797-808.
- van der Helm-van Mil AH, Verpoort KN, Breedveld FC, Toes RE, Huizinga TW. Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res Ther. 2005;7:R949-R958.
- van Gaalen F, Ioan-Facsinay A, Huizinga TW, Toes RE. The devil in the details: The emerging role of anticitrulline autoimmunity in rheumatoid arthritis. J Immunol. 2005;175:5575-5580.
- Rantapaa-Dahlqvist S, de Jong BA, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741-2749.
- Nielen MM, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380-386.
- van Gaalen FA, van Aken J, Huizinga TW, et al. Association between HLA class II genes and autoantibodies to cyclic citrullinated peptides (CCPs) influences the severity of rheumatoid arthritis. Arthritis Rheum. 2004;50:2113-2121.
- Berglin E, Padyukov L, Sundin U, et al. A combination of autoantibodies to cyclic citrullinated peptide (CCP) and HLA-DRB1 locus antigens is strongly associated with future onset of rheumatoid arthritis. Arthritis Res Ther. 2004;6(4):R303-R308.
- Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38-46.
- Lee HS, Irigoyen P, Kern M, et al. Interaction between smoking, the shared epitope, and anti-cyclic citrullinated peptide: A mixed picture in three large North American rheumatoid arthritis cohorts. Arthritis Rheum. 2007;56:1745-1753.
- Hill JA, Southwood S, Sette A, Jevnikar AM, Bell DA, Cairns E. Cutting edge: The conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule. J Immunol. 2003;171:538-541.
- Silman AJ, Newman J, MacGregor AJ. Cigarette smoking increases the risk of rheumatoid arthritis. Results from a nationwide study of disease-discordant twins. Arthritis Rheum. 1996;39:732-735.
- Heliovaara M, Aho K, Aromaa A, Knekt P, Reunanen A. Smoking and risk of rheumatoid arthritis. J Rheumatol. 1993;20:1830-1835.
- Uhlig T, Hagen KB, Kvien TK. Current tobacco smoking, formal education, and the risk of rheumatoid arthritis. J Rheumatol. 1999;26:47-54.
- Stolt P, Bengtsson C, Nordmark B, et al. Quantification of the influence of cigarette smoking on rheumatoid arthritis: Results from a population based case-control study, using incident cases. Ann Rheum Dis.;62:835-841.
- Makrygiannakis D, af Klint E, Lundberg IE, et al. Citrullination is an inflammation-dependent process. Ann Rheum Dis. 2006;65:1219-1222.
- Vossenaar ER, Nijenhuis S, Helsen MM, et al. Citrullination of synovial proteins in murine models of rheumatoid arthritis. Arthritis Rheum. 2003;48:2489-2500.
- Kuhn KA, Kulik L, Tomooka B, et al. Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J Clin Invest. 2006;116:961-973.
- Lundberg K, Nijenhuis S, Vossenaar ER, et al. Citrullinated proteins have increased immunogenicity and arthritogenicity and their presence in arthritic joints correlates with disease severity. Arthritis Res Ther. 2005;7(3):R458-R467.
- Masson-Bessiere C, Sebbag M, Girbal-Neuhauser E, et al. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the alpha- and beta-chains of fibrin. J Immunol. 2001;166:4177-4184.
- Liao KP, Batra KL, Chibnik L, Schur PH, Costenbader KH. Anti-CCP revised criteria for the Classification of Rheumatoid Arthritis. Ann Rheum Dis. 2008;67:1557-1561.