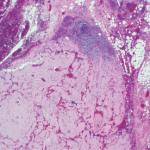
Aβ plaques act primarily as a trigger of other downstream processes, particularly tau aggregation, which mediate neurodegeneration. The major pathogenic effects of Aβ plaques may occur very early in the disease process.7 There may be little benefit in attacking these plaques later in their development. This suspicion has been confirmed by the disappointing results of several clinical trials.8 Targeting critical enzyme inhibitors of plaque formation has failed, too, and a trial using aggregated human Aβ as a therapeutic agent was halted when some participants developed an autoimmune encephalopathy.9 It may be time to abandon the amyloid hypothesis and seek a better explanation for the neuronal destruction caused by AD.
An Unrelenting Adversary or An Unanticipated Ally?
Unfortunately, the deposition of amyloid in any tissue, especially the brain, never relents. The high prevalence of AD has affected countless families as it progressively destroys the brains of some of our loved ones. But it is a condition that is not limited to the aged.
More recently, the term chronic traumatic encephalopathy (CTE) has been applied when the neuropathology findings of boxer’s brain were observed in retired professional football and hockey players, entertainment wrestlers, victims of domestic violence and military veterans exposed to blast and concussive injuries from improvised explosive devices. The cognitive and behavioral symptoms of CTE begin insidiously; first, there may be mood changes such as depression, apathy and irritability, which sadly may end in suicide.11 CTE is characterized by prominent deposition of tau and variable degrees of diffuse amyloid deposition, although the areas of brain involvement seem to differ from what is seen in AD.
Could there be any benefit to amyloid’s presence in our bodies? There may be a silver lining. Unlike other human biological fluids, healthy semen contains multiple types of amyloid fibrils. In a recent study, it was noted that these fibrils inhibited fertilization by immobilizing sperm.12 Interestingly, this immobilization facilitated the uptake and clearance of sperm by macrophages, which are known to infiltrate the female reproductive tract following semen exposure. In the presence of semen fibrils, damaged and apoptotic sperm were more rapidly phagocytosed than healthy ones, suggesting that the deposition of semen fibrils in the female reproductive tract facilitates the clearance of poor-quality sperm.
It’s heartening to learn that nature has found a way to put this pernicious protein to proper use somewhere in our bodies. Who knew? Amyloid as sperm selector; amyloid as our ally.