A 15-year-old girl is fatigued, flushed, and cannot remember anything. She went from As to Fs in school and was seen by her mother putting groceries away in her clothing drawer.
A 12-year-old boy had his appendix out last week. Now he is behaving oddly and his parents have brought him to the emergency room (ER) because he won’t stop swearing—and he undresses in public.
A 15-year-old boy with lupus suddenly has a grand mal seizure. CT of the brain shows a large stroke.
A 17-year-old girl recently diagnosed with lupus nephritis presents to the ER after a bout of diarrhea because her “legs won’t work.” She is admitted to the hospital for the treatment of acute inflammatory demyelinating polyneuropathy.
All of these children presented with dramatic and serious clinical findings and were subsequently diagnosed with neuropsychiatric systemic lupus erythematosus (NPSLE). NPSLE is arguably the least understood manifestation of SLE, as it is associated with a complicated and often baffling range of clinical presentations.1 Since the publication of the new ACR Case Definitions for NPSLE,2 studies have confirmed the presence of NPSLE in as many as 80% of adults with SLE.3 NPSLE syndromes range from diffuse central nervous system (CNS) disorders (e.g., acute confusional state, psychosis, anxiety, and depressive disorders), including cognitive dysfunction, to focal CNS syndromes (e.g., seizures, cerebrovascular disease, chorea, myelopathy, transverse myelitis, demyelinating syndrome, aseptic meningitis, and headaches) and peripheral nervous system disorders (e.g., neuropathies and acute inflammatory demyelination).2
The most common NPSLE symptoms are headache and neurocognitive dysfunction.4 The latter may be transitory or persistent over years and can occur in patients without overt NPSLE or even systemic disease activity. Although risk factors for neuropsychiatric disease are not well defined, a longitudinal study of 123 multiethnic adults with SLE (SALUD study) suggests that a higher level of depression is associated with neurocognitive dysfunction, although the cause and effect of this relationship remains unclear. Furthermore, persistent prednisone use, the presence of antiphospholipid antibodies, a lower education level, and diabetes are associated with cognitive decline in adults with SLE.5
Neuropsychiatric involvement may occur even more commonly in children than in adults with SLE, with up to 95% of the pediatric patients manifesting at least one symptom of NPSLE in some studies.3,6 Often, NPSLE develops early in the disease course, and up to 25% of the patients manifest symptoms within 30 days of diagnosis.7 Cognitive impairment from NPSLE can affect any or all of the following cognitive functions: simple or complex attention, reasoning, executive skills (e.g., planning, organizing, sequencing), memory (e.g., learning, recall), visual-spatial processing, language (e.g., verbal fluency), and psychomotor speed. The severity of cognitive dysfunction in NPSLE ranges from mild impairment to severe dementia. This situation may not be unique to children with lupus, however, because neurocognitive dysfunction occurs with other chronic pediatric diseases, including early-onset insulin-dependent diabetes and sickle cell anemia, and following treatment for childhood malignancies.
The onset of SLE is often peripubertal (10–15 years of age), which is usually after the most rapid period of normal neurocognitive development (5–8 years of age). One could assume, therefore, that the effects of NPSLE are less pronounced and slower in development than those of other chronic childhood diseases that start earlier during a child’s life. However, cognitive maturation continues throughout adolescence into young adulthood, especially with respect to processing speed, response inhibition, and working memory.8 Impaired neurocognitive growth is likely the reason why, on average, the testing scores of pediatric SLE patients are lower than those of their peers, especially for reading comprehension, attention, working memory, and executive functioning.9 Although NPSLE in adults likely affects the expression of known skills only, we have initial evidence that there is impairment of not only existing skills but also of the acquisition of new skills in children with SLE.10
Proposed Etiology of NPLSE Neurobehavioral Syndromes
The etiology of neurocognitive dysfunction in children with SLE is unknown but likely relates to processes proposed to contribute to the development of NPSLE in general. Most likely, the pathogenic etiologies of neurocognitive dysfunction manifestations are multifactorial and may involve autoantibody production, microangiopathy, intrathecal production of proinflammatory cytokines, and atherosclerosis. Small vessel vasculitis, although originally thought to be an important mechanism for NPSLE, is rarely observed on radiological imaging and has not been found on histopathological examination. Instead, pathologic findings in brains of patients with this complication are most consistent with microinfarcts or vasculopathy, with proliferation of intimal cells, fibrin thrombi in small vessel lumens, and perivascular inflammatory infiltrates. Elevated cerebrospinal fluid (CSF) levels of proinflammatory cytokines (ICAM-1, transforming growth factor beta, interferon alpha, and interleukins IL-6, IL-8, and IL-10) have been observed, but the possible mechanisms by which these cytokines could induce brain disease remain unclear. Recent data suggest that increased peripheral cytokine release leads to increased production of nitric oxide and CNS symptoms.
Drug injury, particularly due to the use of corticosteroids, has been proposed as a cause of CNS manifestations in SLE as drugs can induce psychosis and other neuropsychiatric conditions. However, many children have NPSLE at diagnosis, prior to any treatment. Corticosteroid use (dose, duration) in SLE has not been correlated with neurocognitive dysfunction, and corticosteroids may improve mood and cognitive changes due to SLE.
It is becoming increasingly clear that the integrity of the blood–brain barrier is very important in SLE-related neuropathology, and the lack thereof may allow for pathogenic antibodies to attack the brain. Although antineuronal and antiribosomal P antibodies are rare in children, they may be directly pathogenic or an epiphenomenon of NPSLE. Anti-NR2 receptor antibodies recently have been implicated in apoptotic neuronal cell apoptotic death after binding to the N-methyl-D-aspartate receptor but, currently, the role of anti-NR2 antibodies in NPSLE development remains controversial.1 Antiphospholipid antibodies are also implicated in NPSLE. These antibodies likely produce disease through ischemia via thrombi and noninflammatory vasculopathy.
Diagnosis of Neurobehavioral Syndromes in Children
To date, there is no single reliable laboratory test for detecting a neurobehavioral syndrome associated with SLE. The diagnosis of an SLE neurobehavioral syndrome of an individual child is based on clinical, neurological, and rheumatological evaluation, serological testing, brain imaging, and formal neurocognitive testing; the latter is considered the criterion standard for diagnosing cognitive dysfunction in SLE. Often, there is more suspicion than true evidence that SLE might be the cause of a neurobehavioral syndrome in a child.
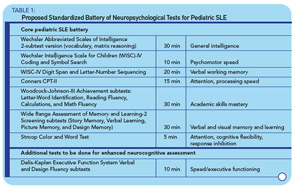
A recent survey among 128 pediatric rheumatologists indicates that 79% would consider performing formal neuropsychological testing for children with a history of overt CNS SLE (stroke, seizure, psychosis) and that 98% of providers would like to obtain formal testing for children with SLE who show academic decline or have cognitive complaints (patient or parent reports). However, besides widespread problems of access to formal neurocognitive testing, such testing is costly and may not be covered by insurance companies. Additionally, for children with SLE, there is no widely accepted standardized battery of neuropsychological tests that could be used as a gold standard for the diagnosis of cognitive dysfunction. This is because the ACR 1-hour Battery is not suitable for the testing of children. The Childhood Arthritis and Related Diseases Research Agenda (CARRA) Lupus Neuropsychiatric Working Group has developed a potential standardized battery, which is currently being tested for validity (see Table 1, below).
Computer-Based Neurocognitive Testing
Undoubtedly, the limited means of diagnosing cognitive dysfunction due to SLE leave many children at risk for remaining untreated for a disease manifestation that significantly decreases patient quality of life and worsens long-term prognosis. Current research is therefore exploring other testing modalities to facilitate recognition of this condition. For example, in adults with SLE, the Automated Neuropsychological Assessment Metrics (ANAM) has been successfully used to test the cognitive functioning of adults with SLE and children as young as 13 years of age (see Figure 1, left).
A pediatric version of the ANAM (Ped-ANAM) software has been designed for use in children who are age 10 years or older; this may be a good screening tool for neurocognitive dysfunction in children with SLE.11 Ped-ANAM testing takes about 30–40 minutes and can be done by the child with little guidance. This test is designed to assess sustained concentration and attention, mental flexibility, spatial processing, cognitive-processing efficiency, arousal/fatigue level, learning, recall, and working memory. More details can be obtained at www.c-shop.ou.edu.
Imaging to Diagnose Neurocognitive Dysfunction
Neuroimaging is a logical means to noninvasively investigate SLE-associated neurocognitive dysfunction, because clinical and laboratory tests have not facilitated timely diagnosis or the understanding of its pathology. Although there has been evidence of cortical and subcortical neuropathology demonstrated by magnetic resonance imaging (MRI), magnetization transfer imaging (MTI), and FLAIR images, correlation of these findings with clinical symptoms has not helped diagnose neurocognitive dysfunction or assess its response to treatment. Findings on conventional MRI have been inconclusive, as lesions are common with SLE in general, and not specifically correlated with, nor sensitive to, change of active neurocognitive dysfunction.
Given the lack of utility of these standard MRI techniques, functional neuroimaging using positron emission tomography and single-photon emission computed tomography have been investigated. Disadvantages of these methods include exposure to radiation and contrast, poor spatial resolution, and a lack of correlation to specific NPSLE syndromes. Metabolic brain studies using magnetic resonance spectrometry (MRS) suggest that the level of cognitive functioning, and NPSLE in general, are associated with both white matter and gray matter changes.12
Other recently used techniques include diffusion-weighted imaging (DWI), which can provide quantitative measurements of brain diffusivity.13,14 Interestingly, DWI shows that patients with SLE who test positive for anti-NR2 antibodies have more severe damage to the amygdala compared with SLE patients without anti-NR2 antibodies. The results from advanced imaging techniques, such as diffusion tensor imaging, functional MRI, and diffusion-weighted imaging, in a group of adult patients with various NPSLE syndromes, suggest that the combination of various imaging techniques provides complementary and synergistic information. However, none of these studies is diagnostic.
Functional MRI (fMRI) is a promising method for recording brain activation patterns associated with specific cognitive tasks, particularly those that examine the neuropathological underpinnings of NPSLE. Standard fMRI studies include the acquisition of serial images while the participant alternates between performing active and control tasks (fMRI paradigms). The image intensity is weighted by the relative oxygenation level of blood hemoglobin. This method has been helpful for delineating changes in brain activity with various chronic diseases involving cognition, such as multiple sclerosis and Alzheimer’s disease. A recent fMRI study compared children with SLE who had normal cognition per formal neuropsychological testing, children with SLE who had neurocognitive dysfunction, and healthy controls for attention, language, and working memory.15
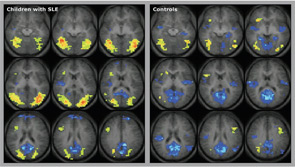
With SLE, brain activation in select cortical areas correlated negatively with a subset of individual neuropsychological test scores in a study of 10 children with SLE. They needed to activate a larger brain area to perform the fMRI tasks, and they were not able to deactivate or suppress brain activation once the task was over (see Figure 2, above). This finding means that, when compared with controls, children with SLE show a statistically significant imbalance between active and inhibitory responses of the brain to fMRI stimuli involving the attention, working memory, and language. Even SLE patients with supposedly normal cognition showed these imbalances, albeit to a lesser degree than those with abnormal formal neuropsychological testing. Differences in brain activation patterns compared with controls suggest that childhood-onset SLE may be associated with abnormalities in white matter connectivity resulting in neuronal network dysfunction, rather than injury of specific gray matter areas.
Treatment of NPSLE
The general management of patients with NPSLE includes symptomatic and immunosuppressive therapies, but evidence for the efficacy of the treatment modalities commonly used is largely limited to uncontrolled clinical trials and anecdotal experience. The key to treatment is to first establish the correct diagnosis by carefully considering all possible etiologies, both SLE-related and those that are not. One important issue in the differential diagnosis of NPSLE is to determine whether current medication side effects could be causing symptoms. Many of the therapies used to treat SLE can cause headache, and some medications can alter mood and cognition, including corticosteroids and anticonvulsant drugs. Infection, thrombosis, and vasculitis can also alter mood and cognitive function. The approach to treatment of pediatric NPSLE is not significantly different from adult disease.
Patients with headache, seizure, and movement disorders receive specific therapies but rarely receive corticosteroids to address these problems, unless the disease manifestations are severe. As in the adult patient, antiphospholipid syndrome, stroke, and vasculitis are considered when patients develop symptoms. Patients with severe manifestations such as psychosis, organic brain syndrome, myelopathy, and some peripheral neuropathies are treated with high-dose steroids (oral and/or IV pulse therapy) and IV pulse cyclophosphamide, although some centers prefer to use azathioprine initially, if the patient is stable.16
Similar to the adult experience, children who have cognitive dysfunction as one of many active disease manifestations often have improvement or even resolution of cognitive abnormalities as other systemic features improve with treatment. For those patients who have cognitive abnormalities that do not prevent everyday function but are noted by family, school, or the patient, evaluation should include neurocognitive and psychiatric assessments.
Patients with cognitive abnormalities associated with major psychiatric diagnoses may require antidepressant or antipsychotic therapy possibly in conjunction with immunosuppressive therapy, as well as counseling. Other possible findings on neurocognitive testing such as attentional problems also can be addressed with medical and cognitive therapy. Children with SLE and a concern of intellectual decline or memory problems have an advantage over adult patients because they are routinely tested in school. It is important to compare previous academic standardized testing to current results, because differences may not be significant and concerns may be due to anxiety or a change in academic demands. Once the patient has an evaluation, the results can be shared with the school. By law, patients who have disabilities can request a 504 or individual education plan to improve school support, including services such as tutors, school psychological evaluations, and specific curricular plans worked out between the patient, parents, medical team, and school team.
Perhaps the greatest challenge for a child with SLE is how to negotiate their social environment. Each child has an individual set of social skills, family, friends, and unique disease features that require different levels of attention and intervention. Significant physical or behavioral changes can alter social acceptance. Education of family, peers, and school faculty is critical to improve social functioning; however, it is not always the desire of the patient and family. Successful reintegration into the social environment is a significant challenge and a unique skill of the pediatric rheumatology team.
Dr. Brunner is associate professor of pediatrics in the divisions of rheumatology and clinical epidemiology, and head of the Cincinnati Children’s Hospital Medical Center Lupus Center at the University of Cincinnati College of Medicine. Dr. Klein-Gitelman is associate professor of pediatrics and head of pediatric rheumatology at the Children’s Memorial Hospital at Northwestern University Feinberg School of Medicine in Chicago.
References
- Hermosillo-Romo D, Brey RL. Neuropsychiatric involvement in systemic lupus erythematosus. Curr Rheumatol Rep. 2002; 4:337-344.
- The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999; 42:599-608.
- Sibbitt WL, Jr., Brandt JR, Johnson CR, et al. The incidence and prevalence of neuropsychiatric syndromes in pediatric onset systemic lupus erythematosus. J Rheumatol. 2002; 29:1536-1542.
- Hanly JG, Fisk JD, McCurdy G, Fougere L, Douglas JA. Neuropsychiatric syndromes in patients with systemic lupus erythematosus and rheumatoid arthritis. J Rheumatol. 2005; 32:1459-1466.
- McLaurin EY, Holliday SL, Williams P, Brey RL. Predictors of cognitive dysfunction in patients with systemic lupus erythematosus. Neurology. 2005; 64:297-303.
- Olfat MO, Al-Mayouf SM, Muzaffer MA. Pattern of neuropsychiatric manifestations and outcome in juvenile systemic lupus erythematosus. Clin Rheumatol. 2004; 23:395-399.
- Harel L, Sandborg C, Lee T, von Scheven E. Neuropsychiatric manifestations in pediatric systemic lupus erythematosus and association with antiphospholipid antibodies. J Rheumatol. 2006; 33:1873-1877.
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004; 75:1357-1372.
- Wyckoff PM, Miller LC, Tucker LB, Schaller JG. Neuropsychological assessment of children and adolescents with systemic lupus erythematosus. Lupus. 1995; 4(3):217-220.
- . Klein-Gitelman MS, Zelko F, Kress A, Hunter S, Wagner-Weiner L. Comparison of neuro-cognitive function in children with pediatric systemic lupus erythematosus (pSLE) and their peers—second year follow-up. Arthritis Rheum. 2002; p. S216.
- . Brunner HI, Ruth NM, German A, et al. Initial validation of the pediatric automated neuropsychological assessment metrics for childhood-onset systemic lupus erythematosus. Arthritis Rheum. 2007;57:1174-1182.
- Mortilla M, Ermini M, Nistri M, DalPozzo G, Falcini F. Brain study using magnetic resonance imaging and proton MR spectroscopy in pediatric onset systemic lupus erythematosus. Clin Exp Rheumatol. 2003; 21:129-135.
- Moritani T, Hiwatashi A, Shrier DA, Wang HZ, Numaguchi Y, Westesson PL. CNS vasculitis and vasculopathy: Efficacy and usefulness of diffusion-weighted echoplanar MR imaging. Clin Imaging. 2004; 28(4):261-270.
- Padovan M, Locaputo A, Rizzo N, Govoni M, Trotta F. The evaluation of neuropsychiatric lupus erythematosus by functional neuroimaging. Preliminary results [in Italian]. Reumatismo. 2004; 56:24-30.
- DiFrancesco M, Holland S, Ris D, et al. Functional magnetic resonance imaging of cognitive function in childhood-onset systemic lupus erythematosus: A pilot study. Arthritis Rheum. 2007; 56:4151-4163.
- Benseler, S. M., E. D. Silverman. Neuropsychiatric involvement in pediatric systemic lupus erythematosus. Lupus. 2007;16:564-571.
