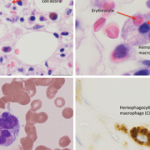
Recently it has been suggested that in patients unresponsive to the combination of steroids and CyA, particularly in those with renal and hepatic impairment, antithymocyte globulin (ATG) might be a safer alternative to etoposide.21 ATG depletes both CD4+ and CD8+ T cells through complement-dependent cell lysis. Mild depletion of monocytes is noted in some patients as well. Although in the reported cases this treatment was tolerated well, one must remember that infusion reactions are frequently reported with the use of ATG and adequate laboratory and supportive medical resources must be readily available if this treatment is used. The utility of biologic drugs in MAS treatment remains unclear. Although, tumor necrosis factor– (TNF-) inhibiting agents have been reported to be effective in occasional MAS patients, other reports describe patients in whom MAS developed while they were on TNF-inhibiting agents. Because, at least in systemic JIA, MAS episodes are often triggered by the disease flare, biologics that neutralize interleukin-1, a cytokine that plays a pivotal role in SJIA pathogenesis, have been tried by some authors. However, as with TNF-inhibiting agents, the results, however, have been conflicting. Based on some success of intravenous immunoglobin treatment in virus-associated reactive HLH, this treatment might be considered in MAS triggered by viral infection. If MAS, however, is driven by EBV infection, one might consider rituximab, a treatment that would eliminates B lymphocytes, the main type of cells harboring EBV virus. This approach has been successfully used in EBV-induced lymphoproliferative disease.22
Prognosis
Although the reported mortality rates of MAS reach 20%, due to increasing awareness it is now diagnosed relatively early and the outcome is improving. A substantial proportion of MAS patients experience recurrent episodes and these patients may require closer monitoring.
Dr. Grom is associate professor of pediatrics in the division of rheumatology at Cincinnati Children’s Hospital Medical Center.
References
- Hadchouel M, Prieur AM, Griscelli C. Acute hemorrhagic, hepatic, and neurologic manifestations in juvenile rheumatoid arthritis: Possible relationship to drugs or infection. J Pediatr. 1985;106:561-566.
- Grom AA. NK dysfunction: A common pathway in systemic onset juvenile rheumatoid arthritis, macrophage activation syndrome, and hemophagocytic lymphohistiocytosis. Arthritis Rheum. 2004; 50:689-698.
- Sawhney S, Woo P, Murray KJ. Macrophage activation syndrome: A potentially fatal complication of rheumatic disorders. Arch Dis Child. 2001; 85:421-426.
- Stephan JL, Kone-Paut I, Galambrun C, et al. Reactive haemophagocytic syndrome in children with inflammatory disorders: A retrospective study of 24 patients. Rheumatology (Oxford). 2001;40:1285-1292.
- Behrens EM, Beukelman T, Paessler M, Cron RQ. Occult macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis. J Rheumatol. 2007; 34:1133-1138.
- Athreya BH. Is macrophage activation syndrome is a new entity? Clin Exp Rheumatol. 2002; 20:121-123.
- Favara BE, Feller AC, Pauli M, et al. Contemporary classification of histiocytic disorders. The WHO Committee On Histiocytic/Reticulum Cell Proliferations. Reclassification Working Group of the Histiocyte Society. Med Pediatr Oncol. 1997;29:157-166.
- Filipovich HA. Hemophagocytic lymphohistiocytosis. Immunol Allergy Clin N Am. 2002;22:281-300.
- Henter JI, Horne A, Arico M, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemopagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124-131.
- Stepp SE, Dufourcq-Lagelouse R, Le Deist F, et al. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286:1957-1959.
- Feldmann J, Callebaut I, Raposo G, et al. MUNC13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3). Cell. 2003; 115:461-473.
- Grom AA, Villanueva J, Lee S, Goldmuntz EA, Passo MH, Filipovich AH. Natural killer cell dysfunction in patients with systemic-onset juvenile rheumatoid arthritis and macrophage activation syndrome. J Peds. 2003. 142;292-296.
- Vastert SJ, van Wijk R, D’Urbano LE, et al. Mutations in the perforin gene can be linked to macrophage activation syndrome in patients with systemic onset juvenile idiopathic arthritis. Rheumatology (Oxford). 2010;49:441-449.
- Menasche G, Feldmann J, Fischer A, de Saint Basile G. Primary hemophagocytic syndromes point to a direct link between lymphocyte cytotoxicity and homeostasis. Immunol Rev. 2005;203:165-179.
- Kagi D, Odermatt B, Mak TW. Homeostatic regulation of CD8+ T cells by perforin. Eur J Immunol. 1999;29:3262-3272.
- Avcin T, Tse SML, Schneider R, Ngan B, Siverman ED. Macrophage activation syndrome as the presenting manifestation of rheumatic diseases in childhood. J Pediatr. 2006; 148:683-686.
- Schaer DJ, Schleiffenbaum B, Kurrer M, et al. Soluble hemoglobin-haptoglobin scavenger receptor CD163 as a lineage-specific marker in the reactive hemophagocytic syndrome. Eur J Haemotol. 2005;74:6-10.
- Kristiansen M, Graversen JH, Jacobsen C, et al. Identification of the hemoglobin scavenger receptor. Nature. 2001;409:198-201.
- Ravelli A, Magni-Manzoni S, Pistorio A, et al. Preliminary diagnostic guidelines for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. J Pediatr. 2005;598-604.
- Ravelli A, De Benedetti F, Viola S, Martini A. Macrophage activation syndrome in systemic juvenile rheumatoid arthritis successfully treated with cyclosporine. J Pediatr. 1996;128:275-278.
- Coca A, Bundy KW, Marston B, Huggins J, Looney RJ. Macrophage activation syndrome: Serological markers and treatment with anti-thymocyte globulin. Clin Immunol. 2009; 132:10-18.
- Balamuth NJ, Nichols KE, Paessler M, Teachey DT. Use of rituximab in conjunction with immunosuppressive chemotherapy as a novel therapy for EBV-associated hemophagocytic lymphohistiocytosis. J Ped Hem/Oncol. 2007;8:569-573.