Editor’s note: The 2020 ACR State-of-the-Art Clinical Symposium, which was originally scheduled to be held in New Orleans March 27–29, was moved to an online format to accommodate social distancing requirements during the COVID-19 pandemic.
Editor’s note: The 2020 ACR State-of-the-Art Clinical Symposium, which was originally scheduled to be held in New Orleans March 27–29, was moved to an online format to accommodate social distancing requirements during the COVID-19 pandemic.
ACR BEYOND LIVE—Cancer and autoimmunity have an interesting and complex relationship, one that is bidirectional and important to understand from all perspectives. For example, many of the medications used to treat rheumatic diseases can potentially increase the risk of malignancy (e.g., cyclophosphamide increases the risk of bladder cancer and hematologic malignancies). Similarly, a new class of oncologic treatments, immune checkpoint inhibitors, can lead to immune-related adverse events, such as pneumonitis, inflammatory arthritis and many other autoimmune phenomena.
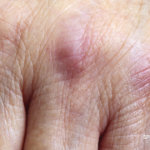
Some autoimmune diseases, such as scleroderma, can potentiate the effects of cancer treatments (e.g., scleroderma can cause exaggerated, radiation-induced fibrosis of the skin and lungs in patients with a history of breast cancer treated with radiation). Moreover, research in recent years has demonstrated that at least two autoimmune diseases—scleroderma and dermatomyositis—likely have a mechanism of cancer-induced autoimmunity and a high cancer risk that make it essential to think about how best to screen for malignancy in patients with these conditions.
At the 2020 ACR State-of-the-Art Clinical Symposium, Ami Shah, MD, MHS, associate professor of medicine, the Division of Rheumatology, and director of clinical and translational research for the Johns Hopkins Scleroderma Center, Baltimore, focused on these two diseases in a discussion of how to use autoantibodies as tools for cancer risk stratification, how to recognize clinical signs of high cancer risk in these patients and how to approach cancer screening in individuals with new-onset disease.
Antibodies & Risk
In 2014, Joseph et al. published a study involving patients with concurrent cancer and scleroderma. The study revealed somatically mutated genes (specifically, the POLR3A gene) in the patients’ tumors that initiated cellular immunity and cross-reactive humoral immune responses, thereby producing antibodies to RPC1 that reacted to the cancer and are known to play an important role in scleroderma itself. This finding implies that acquired immunity helps control naturally occurring cancers and that collateral damage from this mechanism may spur the development of autoimmune disease, such as scleroderma.1
This field of study has helped provide evidence that several autoantibody patterns in scleroderma—namely, the presence of antibodies to RNA polymerase III, antibodies to RNPC3 and triple negativity (no antibodies to centromere, topoisomerase-1 and RNA polymerase III)—are associated with increased risk of cancer.2