 ACR CONVERGENCE 2021—For patients with myositis for whom initial therapies fail to control symptoms, the first step in determining the next therapeutic approach is to determine whether the symptoms are due to active disease or damage.
ACR CONVERGENCE 2021—For patients with myositis for whom initial therapies fail to control symptoms, the first step in determining the next therapeutic approach is to determine whether the symptoms are due to active disease or damage.
If inflammation is persistent, “re-evaluate the diagnosis,” said Ingrid E. Lundberg, MD, PhD, Division of Rheumatology, Department of Medicine, Karolinska Institutet, Solna, Stockholm. However, she continued, “if persisting inflammation and other disorders have been ruled out, change therapy.”
These are the key steps to take when assessing management of a patient with inflammatory myositis refractory to initial therapies, based on newly published algorithms developed to help rheumatologist manage these patients.1 In a session titled Management of Inflammatory Myositis Refractory to Initial Therapies, Dr. Lundberg used a case study to illustrate how the algorithms can be used to help differentiate the cause of persistent inflammation in these patients and treatment considerations based on what is determined (see Figure 1 below for one of the algorithms).
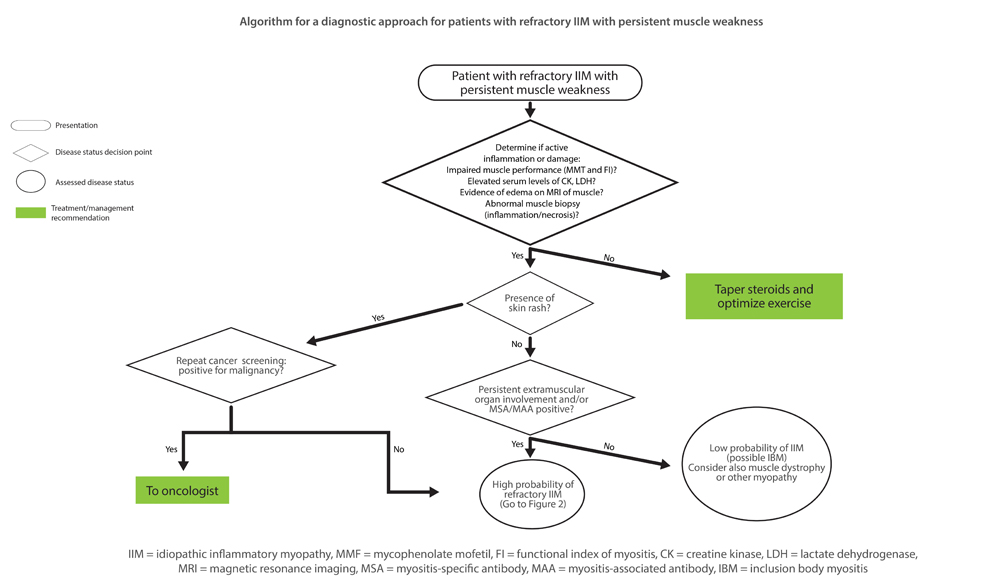
Figure 1
Algorithm for a diagnostic approach for patients with refractory idiopathic inflammatory myopathy (IIM) with persistent muscle weakness. MMT = Manual Muscle Testing; FI = Functional Index in myositis; CK = creatine kinase; LDH = lactate dehydrogenase; MRI = magnetic resonance imaging; MSA = myositis-specific autoantibody; MAA = myositis-associated autoantibody; IBM = inclusion body myositis.
Reprinted by permission from Lundberg IE. Expert perspective: Management of refractory inflammatory myopathy. Arthritis Rheumatol.1
Dr. Lundberg opened the session by emphasizing the heterogeneous and often systemic nature of inflammatory myositis that can affect more than the muscles, and include the skin, lung, joints and heart. However, she said, autoantibodies detected in past decades have identified more homogenous disease groups associated with certain myositis-specific autoantibodies (MSA) found in both adult and juvenile disease. These MSAs were found in up to 50% of some cohort studies.
To date, few randomized controlled trials are available to guide treatment. In the absence of such evidence, she recommends turning to expert opinion that provides guidance on first-, second- and third-line therapies.2 All three lines of therapy include glucocorticoids, with or without intravenous immunoglobulin (IVIG) therapy, but they differ with regard to biologic agents suggested.
For patients for whom first-line therapy with methotrexate or azathioprine fails, Dr. Lundberg said the recommended second-line therapy is mycophenolate mofetil (MMF), tacrolimus or cyclosporine or combination therapy of methotrexate and azathioprine.
Rituximab, cyclophosphamide, repository corticotropin injection (RCI) or other biologic agents are recommended as third-line therapy when patients don’t have a therapeutic response to a second-line treatment.
To assess response to treatment, several outcome measures are important, including disease activity (i.e., global assessments, muscle strength, physical function, lab measures, and extra-skeletal muscle disease activity), damage and quality of life. Improvement in both adults and children is defined as a 20% improvement in three or more of any of the core set of measures, with no more than two worse by 25% or more.
Using a case study, Dr. Lundberg then described how to implement treatment algorithms for refractory inflammatory myositis based on the treatments and response to treatments detailed above. If active disease is found, re-evaluate the diagnosis, she said. If active myositis is confirmed, switch treatment or add another immunosuppressive treatment, biologic or IVIG.
She emphasized the importance of including physical exercise with immunosuppressive treatment, as well as tailoring treatment according to clinical phenotype and serotype.
Overall, Dr. Lundberg underscored the need for new therapies. “Unfortunately, there are few controlled trials in inflammatory myositis and its subgroups, so we lean on case studies and expert opinion,” she said.
Treatment Landscape
Océane Landon-Cardinal, MD, FRCPC, a rheumatologist in the Centre Hospitalier de l’Université de Montréal (CHUM) followed with a presentation on emerging therapies and potential novel therapeutic targets. Describing evidence on the key role played by type I interferon in dermatomyositis (DM), she discussed evidence to date from two trials assessing the efficacy of Janus kinase (JAK) inhibitors and toll-like receptor 7/8/9 antagonist in this setting.
One small study suggests that tofacitinib may be effective for patients with DM. At 12 weeks, a minimal to moderate improvement in disease activity was experienced in all 10 subjects according to the 2016 ACR/EULAR myositis response criteria, including a significant improvement on skin disease activity. This improvement was sustained at two years in the extension study.3
Another study looking at the potential of a toll-like receptor 7/8/9 antagonist (IMO-8400) offered less promise, with results showing that this agent conferred no significant difference in skin disease activity compared with placebo in a cohort of 30 patients with DM.4
Other agents in the pipeline to treat DM include baricitinib, a JAK inhibitor; PF-06823859, an anti-interferon β monoclonal antibody; apremilast, a PDE-4 inhibitor; abatacept, a T cell co-stimulation blockade; lenabasum, a cannabinoid-2 agonist; tocilizumab, an anti-interleukin (IL) 6 receptor antibody; octagam 10%, a 10% solution of a human intravenous immunoglobulin; and ravulizumab, a complement inhibitor.
Dr. Landon-Cardinal discussed current investigations into targets and treatments in the pipeline for immune-mediated necrotizing myopathy (IMNM), inclusion-body myositis (IBM) and myositis-associated interstitial lung disease. Of these, she described the first trial looking at a complement inhibitor (zilucoplan) to treat IMNM, as well as published data from two randomized trials assessing the efficacy of targeted therapies for IBM.5,6
Results of a trial assessing the efficacy and safety of sirolimus, an mTOR pathway inhibitor, for IBM found that sirolimus conferred no difference in quadriceps strength—the study’s primary end point—at 52 weeks, but was associated with a significant difference in six-minute walk distance, forced vital capacity, Health Assessment Questionnaire–Disability Index (HAQ-DI) and thigh fat fraction—secondary end points. Dr. Landon-Cardinal noted the evidence in these positive end points supports further investigation with another randomized trial. She also noted that 45% of patients treated with sirolimus experienced serious adverse events, such as mouth ulcers, aseptic pneumonia and lower limb edema.5
Dr. Landon-Cardinal also discussed a long-term extension study on the use of bimagrumab, a myostatin inhibitor, for IBM that showed a good safety profile with only rare serious events but no difference from placebo in efficacy (i.e., six-minute walk distance), which resulted in ending the study early.6
Along with a planned phase 3 trial of sirolimus, other agents in the pipeline to treat IBM include arimoclomol, an inducer of the heat shock protein response, and ABC008, an anti-KLRG1 antibody.
Mary Beth Nierengarten is a freelance medical journalist based in Minneapolis.
References
- Lundberg IE. Expert perspective: Management of refractory inflammatory myopathy. Arthritis Rheumatol. 2021 Aug;73(8):1394–1407.
- Oddis CV, Aggarwal R. Treatment in myositis. Nat Rev Rheumatol. 2018 May;14(5):279–289.
- Paik JJ, Casciola-Rosen L, Shin JY, et al. Study of tofacitinib in refractory dermatomyositis: An open-label pilot study of ten patients. Arthritis Rheumatol.2021 May;73(5):858–865.
- Kim YJ, Schiopu E, Dankó K, et al. A phase 2, double-blinded, placebo-controlled trial of toll-like receptor 7/8/9 antagonist, IMO-8400, in dermatomyositis. J Am Acad Dermatol.2021;84(4):1160–1162.
- Benveniste O, Hogrel JY, Belin L, et al. Sirolimus for treatment of patients with inclusion body myositis: A randomized, double-blind, placebo-controlled, proof-of-concept, phase 2b trial. Lancet Rheumatol. 2021;3(1):e340–e348.
- Amato AA, Hanna MG, Machado PM, et al. Efficacy and safety of bimagrumab in sporadic inclusion body myositis: Long-term extension of RESILIENT. 2021 Mar 23;96(12):e1595–e1607.
EuroMyositis Register
Dr. Lundberg urged rheumatologists to join the EuroMyositis Register, an international collaboration to help advance myositis research. The register can also be used as a tool to make decisions on treatment for patients, she said. As of September 2021, more than 5,900 patients from 23 centers worldwide had been entered in the registry.
Learn more at www.euromyositis.eu, or contact Dr. Lundberg via email at [email protected] or Hector Chinoy, PhD, FRCP, BMBS, MSc, BMedSci, at [email protected].




