The Case
A 64-year-old previously healthy man was admitted to the hospital with a 1.5-month history of severe headache, confusion, personality change, and progressive cognitive decline. He had no previous medical problems. Results of the physical examination showed ataxia. The mental status exam revealed impairment of cognitive functions. Laboratory test findings were unremarkable. The erythrocyte sedimentation rate was 2 mm/hour. Tests with normal or negative findings included serum antinuclear antibodies, antineutrophil cytoplasmic antibodies, antibodies against double-stranded DNA, cryoglobulins, anticardiolipin antibodies, blood coagulation studies, tuberculin skin test, serology tests (for hepatitis B, hepatitis C, and human immunodeficiency virus), and a fungal serology survey (Coccidioides, Histoplasma, and Blastomyces). Cerebrospinal fluid (CSF) protein concentration was 97 mg/dl (normal, 14–45 mg/dl), erythrocyte cell count was 50/ul, and white blood cell count was 23/ul (90% lymphocytes, 10% monocytes). Cytologic findings were normal. Additional CSF tests with negative results included the venereal disease research laboratory test, Cryptococcus antigen test, fungal and bacterial cultures, and polymerase chain reaction assays for herpes simplex and zoster viruses, Epstein-Barr virus, cytomegalovirus, Toxoplasma gondii, and Borrelia burgdorferi.
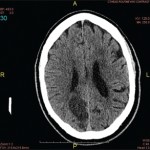
Findings on a chest radiograph, transesophageal echocardiogram, and duplex carotid ultrasonogram were unremarkable. Initial contrast-enhanced magnetic resonance imaging (MRI) of the brain showed diffuse bilateral leptomeningeal enhancement involving the cerebrum and the cerebellum, multiple infarcts, and patchy T2-weighted white matter signal abnormality. Conventional cerebral angiography was normal.
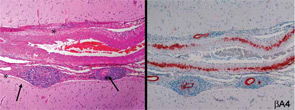
Because of the clinical history and MRI findings, a diagnosis of primary central nervous system vasculitis (PCNSV) was considered, and an open brain biopsy was performed. Pathology showed granulomatous leptomeningeal and intraparenchymal vasculitis. Infarcts and vascular beta-A4 amyloid deposition consistent with cerebral amyloid angiopathy (CAA) were also present (see Figure 1). Stains of biopsy specimens were negative for fungal and mycobacterial organisms.
PCNSV associated with CAA was diagnosed, and the patient was treated with oral prednisone (initial dosage, 40 mg/day) and monthly pulse intravenous injections of cyclophosphamide (CYC) (1.7 gm/month) for 14 months. The patient’s neurologic state progressively improved in the first three months. He was much less confused, his headache resolved, and he was able to perform many of his previous activities. At the time of follow-up, cranial MRI showed complete resolution of leptomeningeal enhancement and no new infarcts. At his final visit 12 months later, the patient was no longer receiving prednisone or immunosuppressives and had no recurrence of symptoms attributable to vasculitis. He had only a minimal disability and conducted normal activities.
PCNSV
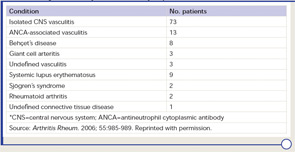
PCNSV is a poorly understood form of vasculitis that is limited to the brain and spinal cord and occurs in both adults and children.1,2 PCNSV represents the most frequent form of vasculitis involving the CNS (see Table 1).3 Modern recognition of PCNSV as a separate entity is generally dated to the mid-1950s when Cravioto and Feigin described several cases with a “non-infectious granulomatous angiitis” with a predilection for the nervous system.4 PCNSV is a rare condition. The only reported incidence rate estimate for PCNSV derives from Olmsted County, Minn., and is 2.4 cases per 1,000,000 person-years. Men and women are similarly affected. The median age at diagnosis is approximately 50 years.1
Diagnosis
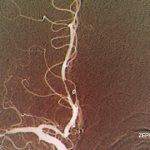
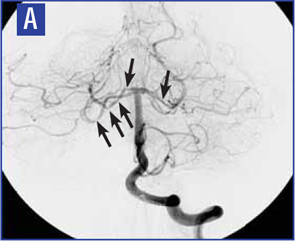
Diagnostic criteria for PCNSV were proposed by Calabrese and Mallek in 1988 on the basis of clinical experience and literature evidence (see Table 2).5 Angiographic changes indicating a high probability of vasculitis include alternating areas of smooth walled narrowing and dilated cerebral arteries, or occlusions, affecting multiple cerebral vessels in the absence of proximal vessel atherosclerosis or other recognized abnormalities (see Figure 2A). A single abnormality in multiple arteries or multiple abnormalities in a single vessel are less consistent with PCNSV.1
Because brain or spinal cord biopsy has been considered a more invasive procedure, angiography has become, by default, the more commonly used method for confirming the diagnosis in patients with suggestive clinical findings. Of note, angiographic changes typical of vasculitis may be seen in nonvasculitic conditions such as vasospasm, CNS infections, lymphomas, cerebral arterial emboli, and atherosclerosis.6,7
Among pathologically documented cases, cerebral angiography may be normal because vascular abnormalities in small vessels can be beyond the resolution of angiography.8 Overall, the sensitivity of angiography varies between 40% and 90%, while the specificity has shown to be as low as 30%.1,9 It is important to emphasize that the diagnosis of PCNSV should not be based on positive angiography alone and that angiography results should always be interpreted in conjunction with clinical, laboratory, and MRI findings.
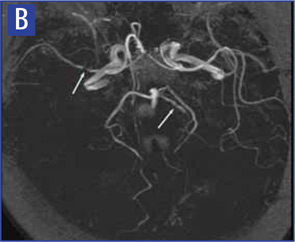
Magnetic resonance angiography (MRA) is a useful modality in the initial investigation of suspected PCNSV (see Figure 2B). However, MRA is less sensitive than conventional angiography in detecting lesions that involve the posterior circulation and distal vessels.1,10 In cases with normal MRA but a high suspicion for PCNSV, cerebral angiography should be performed.

PCNSV is unlikely in the presence of a normal MRI. Several studies have reported MRI abnormalities in close to 100% of cases.1,9 Abnormal findings on MRI are nonspecific and may include cortical and subcortical infarction, parenchyma and leptomeningeal enhancement, intracranial hemorrhage, tumor-like mass lesions, and nonspecific areas of increased signal intensity on FLAIR or T2-weighted images.
Wall thickening and intramural contrast enhancement could be specific findings in patients with active cerebral vasculitis affecting large arteries. Occasionally, enhancement may be marked and extend into the adjacent leptomeningeal tissue (perivascular enhancement).11,12 High-resolution 3T contrast–enhanced MRI may be able to differentiate enhancement patterns of intracranial atherosclerotic plaques (eccentric), inflammation (concentric), and other wall pathologies. However, the sensitivity and specificity of this technique remains to be determined.13
Cerebral and meningeal biopsy remains the gold standard for the diagnosis of PCNSV.6,14 In expert hands, the procedure is relatively safe, with a risk of persistent complications in only about 1% of cases. Diagnostic histopathological features of PCNSV include transmural vascular inflammation affecting small and medium-sized leptomeningeal and parenchymal arterial vessels.14 Granulomatous vasculitis is the most common pattern of vasculitis. Beta-4 amyloid deposition is present in almost 50% of biopsies with this pattern (see Figure 1) but not seen in other histopathological patterns. Lymphocytic vasculitis is the second most predominant pattern. Necrotizing vasculitis is the least frequently seen pattern. In such cases, the appearance is similar to that seen in polyarteritis nodosa, with transmural fibrinoid necrosis.
Vasculitis affects vessels in a skipped and segmental pattern; therefore, because of sampling error, a negative biopsy does not exclude the diagnosis of vasculitis. Biopsy of a radiographically abnormal area is preferable to random sampling of the nondominant frontal lobe or temporal tip. Miller et al showed that 78% of the targeted biopsies were diagnostic, whereas none of the blind biopsies demonstrated vasculitis.14 Stereotactic guidance may be used for deeper lesions but is usually unnecessary for the commonly biopsied, more superficial lesions.
Clinical Manifestations and Laboratory Tests
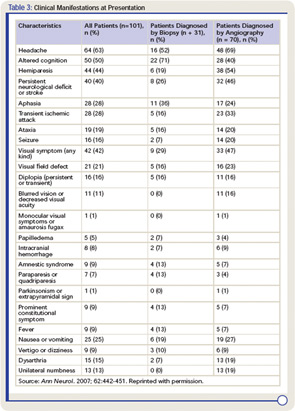
Clinical manifestations of PVCNS at the time of diagnosis are listed in Table 31 These findings are nonspecific, and multiple manifestations are usually present. Although the onset of the disease may be acute, it is more frequently insidious and slowly progressive.
Headache, which is the most common symptom of PCNSV, may be generalized or localized, often slowly worsening, and may spontaneously remit for periods. Cognitive impairment is the second most frequent manifestation. Focal neurological manifestations are present in a large proportion of patients. Other manifestations such as ataxia, seizures, and intracranial hemorrhage are less frequent. Systemic symptoms such as fever and weight loss are uncommon. Symptoms related to spinal cord involvement may occasionally be the presenting manifestation.
Laboratory blood tests including acute phase reactants are usually normal in PCNSV.
CSF analysis is abnormal in 80% to 90% of cases.1 Changes include a mildly increased leukocyte count and total protein concentration. CSF analysis should include appropriate stains, culture, serologic and molecular tests, and flow cytometry studies to exclude infections or malignancy.
Special Subsets
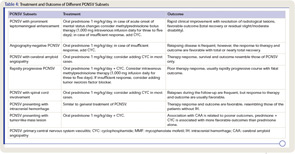
Several clinical subsets of PCNSV, which may differ in terms of prognosis and optimal management, have been identified (see Table 4).
Spinal cord involvement has been documented in 5% of patients, but it is rarely the only manifestation.15 The thoracic cord is the predominantly affected site. A careful medical evaluation must be performed to confirm the diagnosis of PCNSV and to exclude other conditions associated with acute or subacute transverse myelitis.
Angiography-negative, biopsy-positive PCNSV can occur in patients with vasculitis limited to small vessels whose detection is beyond the resolution of conventional angiography.8 These patients often have a cognitive dysfunction at presentation, marked elevation in CSF total protein level, meningeal or parenchymal enhancing lesions on MRI, respond favorably to treatment, and have a good outcome.
Around a quarter of PCNSV biopsy-positive patients have evidence of vascular deposits of amyloid.16 Brain biopsies show granulomatous vasculitis and vascular deposits of amyloid β peptide (see Figure 1). The relationship between these cases and older patients with amyloid deposition but no vascular inflammation (CAA) is uncertain. Patients with PCNSV with amyloid deposits are older at presentation than those with PCNSV without amyloid but younger than patients with CAA and no vasculitis. Patients with PCNSV with amyloid have a high frequency of cognitive dysfunction at presentation and enhancing meningeal lesions on MRI. As the case described above illustrates, these patients usually have a monophasic disease course and generally respond well to immunosuppressive treatment.
PCNSV may present with prominent leptomeningeal enhancement on MRI.17 These patients have an acute clinical onset, cognitive dysfunction is frequently present, and cerebral angiography or MRA are negative. CNS biopsies show a granulomatous vascular inflammation, often associated with CAA. Almost all patients respond to corticosteroid therapy (alone or combined with immunosuppressive agents), with resolution of MRI enhancement and an overall favorable course.
Rapidly progressive PCNSV represents the most severe end of the clinical spectrum of this form of vasculitis.18 Patients have a rapidly progressive course and often a fatal outcome. In these patients, disease is characterized by bilateral, multiple, large cerebral vessel lesions on angiograms and multiple bilateral cerebral infarctions. The predominant histopathological pattern is granulomatous and/or necrotizing. These patients respond poorly to the traditional immunosuppressive treatment.
Approximately 4% of PCNSV patients present with a solitary tumor-like mass lesion.19
An association with CAA was observed in 29% of these patients. Excision of the lesion may be curative; however, in some patients, aggressive immunosuppressive therapy has resulted in a favorable outcome, obviating the need of surgery.
Intracranial hemorrhage (IH) is a presenting manifestation in 11% to 12.2% of patients.20 Intracerebral hemorrhage is the most common, followed by subarachnoid hemorrhage. These patients have less frequently altered cognition, a persistent neurologic deficit, or MRI evidence of cerebral infarctions during the disease course. Necrotizing vasculitis is the predominant histopathologic pattern on biopsy.
Differential Diagnosis
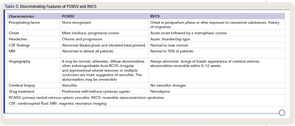
Given the different therapeutic and prognostic implications, it is essential to differentiate between PCNSV and both its mimics as well as secondary causes of CNS vasculitis. The most common mimics of PCNSV are reversible cerebral vasoconstriction syndromes. Grouped under this category are several disorders with different appellations (such as Call-Fleming syndrome, postpartum angiopathy, migrainous vasospasm, and drug-induced cerebral vasculopathy) producing symptoms by vasoconstriction rather than vasculitis.21 Differentiation is crucial because immunosuppressive therapy beyond a short course of prednisone is not warranted for syndromes caused by vasoconstriction (see Table 5).
Infectious agents reported in association with CNS vasculitis include varicella zoster virus, human immunodeficiency virus, treponema pallidum, hepatitis virus C, parvovirus B19, cytomegalovirus, Mycoplasma pneumoniae, B burgdorferi, Mycobacterium tuberculosis, Bartonella, and Rickettsiae.
Fungal infections (aspergillosis, mucormycosis, coccidioidomycosis, and candidiasis) and subarachnoid cysticercosis have also been implicated in some cases.
CNS vasculitis has also been observed in antineutrophil cytoplasmic antibody–associated vasculitis and Behçet’s syndrome. It has also been reported, although rarely, in polyarteritis nodosa, Henoch-Schonlein purpura, Kawasaki disease, giant cell arteritis, and Takayasu arteritis.
CNS vasculitis is an uncommon manifestation of neurosystemic lupus erythematosus, rheumatoid arthritis, primary Sjögren’s syndrome, dermatomyositis, and mixed connective tissue disease.
PCNSV has been associated with lymphoma, particularly Hodgkin’s disease.
Secondary CNS vasculitis also has been described in patients with neurosarcoidosis, inflammatory bowel disease, graft-versus-host disease, and with the use of some drugs (cocaine, amphetamine, ephedrine, and phenylpropanolamine).
Treatment
There are no randomized clinical trials of the medical management in PCNSV. Therefore, treatment for PCNSV has been derived from therapeutic strategies used to treat other vasculitides, anecdotal reports, and PCNSV cohort studies. The earliest reports suggested a poor prognosis with a fatal outcome in the majority of the cases, and transient or doubtful efficacy of glucocorticoids. In 1983, Cupps et al first found CYC in combination with corticosteroids to be effective in PCNSV.22 The most recent PCNSV cohort studies have described a more favorable course of PCNSV. In a study of 101 patients, a favorable response to glucocorticoids alone or in combination with CYC was achieved in 81% of the cases.1
Glucocorticoid therapy should be initiated as soon as the diagnosis of PCNSV is established. We recommend an initial dose of prednisone of 1 mg/kg/day (or equivalent) as a single or divided dose. If the patient does not respond promptly, CYC should be started. A short course of oral CYC for three to six months can also be considered for PCNSV to induce remission, as in other vasculitis. Intravenous pulses of CYC are probably safer than daily oral therapy, although it is unclear whether the two regimes differ in terms of efficacy in PCNSV. After initial treatment with prednisone and cytoxan, one can consider a lower-risk immunosuppressant such as azathioprine, methotrexate, or mycophenolate mofetil (MMF) for maintenance of remission. A treatment course of 12 to 18 months appears adequate in the majority of cases.
Tumor necrosis factor–α (TNF-α) blockers and MMF have successfully been used to treat patients with PCNSV resistant to glucocorticoids and immunosuppressives.23,24 Anti-CD20 therapy with rituximab has been used successfully in refractory granulomatosis with polyangiitis (Wegener’s) involving the CNS, suggesting a possible role for this drug in treating PCNSV.25
There is emerging evidence that therapy of PCNSV can differ among patients and that a uniform approach is not indicated (see Table 4). Relapses or recurrences were recorded in only 26% of the Mayo Clinic series.1 Patients with relapsing disease required longer therapy but otherwise had outcomes similar to those without relapses.
Therapy appears to be associated with a favorable outcome in most patients. In the Mayo Clinic series, most patients who had low disability at the time of diagnosis continued to have low disability at last follow-up; in contrast, most of the 22 patients with severe disability at diagnosis had less disability at follow-up.1
Serial MRI and MRA (four to six weeks after the beginning of treatment, then every three to four months during the first year of treatment, or in case of new neurological deficit), as well as repeat careful neurological examinations are useful to monitor the disease course. In patients with stable imaging but worsening clinical symptoms, a repeat spinal fluid exam and repeat angiography may be indicated.
Our understanding of PCNSV and the delineation of its spectrum and subsets has advanced, but further study is needed to clarify methods of diagnosis and optimal management.
Acknowledgments
We thank Drs. John Huston III, Teresa J.H. Christianson, Kenneth T. Calamia, James F. Meschia, Caterina Giannini, and Dylan Miller for their collaboration in clinical studies on primary central nervous system vasculitis; Dr. Huston for providing angiographic and MRI documentation, and Drs. Giannini and Miller for providing histopathological documentation.
Dr. Salvarani is director of the Unit of Rheumatology at Arcispedale S. Maria Nuova in Reggio Emilia, Italy. Dr. Brown is chair of neurology at the Mayo Clinic in Rochester, Minn. Dr. Hunder is professor emeritus at the Mayo Clinic College of Medicine in Rochester, Minn.
References
- Salvarani C, Brown RD Jr, Calamia KT, et al. Primary central nervous system vasculitis: Analysis of 101 patients. Ann Neurol. 2007;62:442-451.
- Benseler SM, Silverman E, Aviv RI, et al. Primary central nervous system vasculitis in children. Arthritis Rheum. 2006;54:1291-1297.
- Salvarani C, Giannini C, Miller DV, Hunder G. Giant cell arteritis: Involvement of intracranial arteries. Arthritis Rheum. 2006; 55:985-989.
- Cravioto H, Feigin I. Noninfectious granulomatous angiitis with a predilection for the nervous system. Neurology. 1959;9:599-609.
- Calabrese LH, Mallek JA. Primary angiitis of the central nervous system. Report of 8 new cases, review of the literature, and proposal for diagnostic criteria. Medicine (Baltimore). 1988; 67:20-39.
- Moore PM. Diagnosis and management of isolated angiitis of the central nervous system. Neurology. 1989;39:167-173.
- Kadkhodayan Y, Alreshaid A, Moran CJ, Cross DT 3rd, Powers WJ, Derdeyn CP. Primary angiitis of the central nervous system at conventional angiography. Radiology. 2004;233:878-882.
- Salvarani C, Brown RD Jr, Calamia KT, et al. Angiography-negative primary central nervous system vasculitis: A syndrome involving small cerebral vessels. Medicine (Baltimore). 2008; 87:264-271.
- Duna GF, Calabrese LH. Limitations of invasive modalities in the diagnosis of primary angiitis of the central nervous system. J Rheumatol. 1995;22:662-667.
- Eleftheriou D, Cox T, Saunders D, Klein NJ, Brogan PA, Ganesan V. Investigation of childhood central nervous system vasculitis: Magnetic resonance angiography versus catheter cerebral angiography. Dev Med Child Neurol. 2010;52:863-867.
- Küker W, Gaertner S, Nagele T, et al. Vessel wall contrast enhancement: A diagnostic sign of cerebral vasculitis. Cerebrovasc Dis. 2008;26:23-29.
- Salvarani C, Brown RD Jr, Huston J 3rd, Hunder GG. Prominent perivascular enhancement in primary central nervous system vasculitis. Clin Exp Rheumatol. 2008;26(3 Suppl 49):S111.
- Swartz RH, Bhuta SS, Farb RI, et al. Intracranial arterial wall imaging using high-resolution 3-tesla contrast-enhanced MRI. Neurology. 2009;72:627-634.
- Miller DV, Salvarani C, Hunder GG, et al. Biopsy findings in primary angiitis of the central nervous system. Am J Surg Pathol. 2009;33:35-43.
- Salvarani C, Brown RD Jr, Calamia KT, et al. Primary CNS vasculitis with spinal cord involvement. Neurology. 2008;70:2394-2400.
- Salvarani C, Brown RD Jr, Calamia KT, et al. Primary central nervous system vasculitis with prominent leptomeningeal enhancement: A subset with a benign outcome. Arthritis Rheum. 2008; 58:595-603.
- Salvarani C, Brown RD Jr, Calamia KT, et al. Primary central nervous system vasculitis: Comparison of patients with and without cerebral amyloid angiopathy. Rheumatology (Oxford). 2008;47:1671-1677.
- Salvarani C, Brown RD Jr, Calamia KT, et al. Rapidly progressive primary central nervous system vasculitis. Rheumatology (Oxford). 2001;50:349-358.
- Molloy ES, Singhal AB, Calabrese LH. Tumour-like mass lesion: An under-recognised presentation of primary angiitis of the central nervous system. Ann Rheum Dis. 2008; 67:1732-1735.
- Salvarani C, Brown RD Jr, Calamia KT, et al. Primary central nervous system vasculitis presenting with intracranial hemorrhage. Arthritis Rheum. In press.
- Ducros A, Bousser MG. Reversible cerebral vasoconstriction syndrome. Pract Neurol. 2009;9:256-267.
- Cupps TR, Moore PM, Fauci AS. Isolated angiitis of the central nervous system. Prospective diagnostic and therapeutic experience. Am J Med. 1983;74:97-105.
- Salvarani C, Brown RD Jr, Calamia KT, et al. Efficacy of tumor necrosis factor alpha blockade in primary central nervous system vasculitis resistant to immunosuppressive treatment. Arthritis Rheum. 2008;59:291-296.
- Sen ES, Leone V, Abinun M, et al. Treatment of primary angiitis of the central nervous system in childhood with mycophenolate mofetil. Rheumatology (Oxford). 2010;49:806-811.
- Holle JU, Gross WL. Neurological involvement in Wegener’s granulomatosis. Curr Opin Rheumatol. 2011;23:7-11.