Individuals with an excess of nutrition and an excess of soluble urate are often diagnosed with gout. They are diagnosed when their excess urate forms monosodium urate (MSU) crystals that deposit in their articular and soft tissue. The deposits can remain quiescent or trigger the inflammation that is characteristic of gout. Scientists still don’t fully understand why a quiescent urate crystal deposit turns gouty. However, several factors have been identified as playing a role in the pathology of gout. These include the physical characteristics of the MSU crystals, stability of the deposit and systemic triggers from outside the joint. The formed gouty tophi have been characterized, and include not only the MSU crystal deposits, but also macrophages, mast cells, B cells, T cells and other plasma cells.
Most patients with gout have excess body weight, and many have been diagnosed with metabolic syndrome. Scientists have learned that the inflammation that is characteristic of gout is primarily mediated by complement and macrophages, and includes the expression of nuclear factor (NF)-ҡB-dependent proinflammatory cytokines, such as pro-interleukin (IL) 1β, neutrophil chemotactic chemokines CXCL8 (IL-8) and CXCL1. The inflammatory response also centers on activation of the NLRP3 inflammasome and consequent release of IL-1β.
Metabolic Biosensor Enters the Story
A new study suggests the nutritional biosensor AMP-activated protein kinase (AMPK) is the link between nutritional excess and the inflammation that leads to gout.1 AMPK is widely expressed throughout the body and is a driver of stamina. It is activated by stressors that increase the AMP:ATP ratio, such as nutrient deprivation, hypoxia and exercise. As would be expected, individuals who are more physically fit have higher AMPK activity. Previous studies have also shown that excesses in soluble urate suppress tissue AMPK activity. Thus, AMPK has been loosely connected to gout pathophysiology.
Patients with gout know that nutritional excesses and alcohol excesses can trigger gout flares. Metabolic syndrome, obesity, alcohol and multiple nutritional stressors also decrease tissue AMPK activity. In contrast, lifestyle changes, as well as drugs, can activate AMPK. New research adds colchicine to the list of indirect AMPK-promoting drugs. The list includes methotrexate, high-dose aspirin and metformin.
AMPK may thus represent a target for improving efficacy of prophylaxis and treatment of gouty inflammation. Patients may, therefore, be able to improve their gout management by activating their AMPK through exercise and calorie restriction. This new way of thinking about gout expands patient management options beyond the specific dietary restrictions that are designed to decrease the formation of MSU crystals.
Colchicine Drives up AMPK Activity
Colchicine is frequently prescribed to patients with gout. It is also known to affect AMPK by inhibiting microtubule polymerization. Colchicine regulates LKB1 activity in macrophages and research has demonstrated that most of its antiinflammatory effects are dependent on the presence of LKB1. The latest research suggests, however, that colchicine is an effective treatment for gout because it activates the nutritional biosensor AMPK.
Specifically, colchicine activates AMPK in macrophages, and its ability to increase and sustain AMPK activity appears to underlie its ability to limit MSU crystal inflammation both in vitro and in vivo. AMPK does this by transducing the multiple antiinflammatory effects of colchicine in macrophages. Moreover, AMPK is able to shift the overall differentiation of macrophages to an M2 phenotype, which is characterized by macrophages that are less inflammatory and, instead, behave more in a clean-up mode.
Latest Research on AMPK & Gout
Yun Wang, PhD, a postdoctoral fellow in the Department of Medicine at the University of California in San Diego (UCSD), and colleagues published the results of their investigation of AMPK online Oct. 31 in the Annals of the Rheumatic Diseases.1 The team investigated whether AMPK activation is able to limit MSU crystal-induced inflammation. Specifically, they examined the effects of pharmacological activation of AMPK on MSU crystal-induced inflammatory responses. The investigators focused their efforts on cultured macrophages and then expanded their work to a murine model for gout. They investigated the effect of the highly selective AMPK activator, A-769662, and compared the results with treatment with colchicine. The researchers used Western blot and quantitative RT-PCR to examine phosphorylation (activation) of AMPKα Thr172, NLRP3 mRNA expression, caspase-1 cleavage, and IL-1β maturation. They also measured IL-1β and CXCL1 production via ELISA.
Wang et al began their investigation with bone marrow-derived macrophages (BMDMs) obtained by flushing the bone marrows derived from 7–8-week-old mice. They found that MSU crystals (verified to be free of lipopolysaccharide) suppressed phosphorylation of AMPKα in these cells. Their results were consistent with previous research that had demonstrated that some inflammatory stimuli inhibit phosphorylation of the α subunit of heterotrimeric AMPK—the subunit that is critical for AMPK activity.
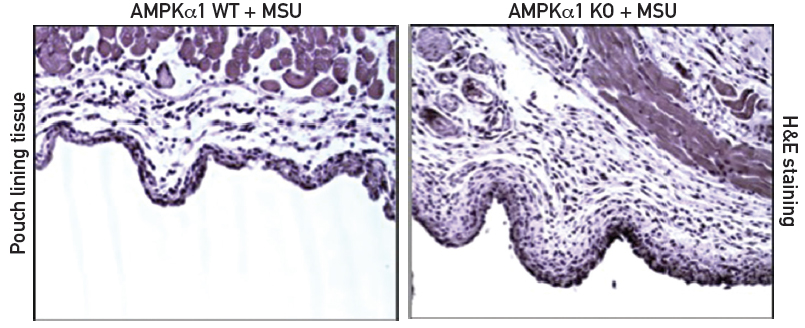
The investigators then turned their attention to AMPKα1 knockout mice. These C57BL/6/129 mice lack one (AMPKα1) of two isoforms of AMPK that are expressed in macrophages. AMPKα1 is the isoform that is predominantly activated in inflammatory cells. As expected, the BMDM cells from knockout mice have decreased expression of total AMPKα when compared to BMDM cells from wild-type mice. The knockout mice are phenotypically normal, but they respond differently than wild-type mice to biological challenges. Specifically, AMPKα1 deficiency significantly enhanced the inflammatory response to MSU crystals both in vitro and in vivo (see Figure 1).
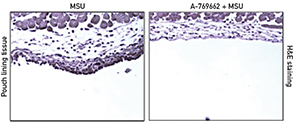
The investigators used subcutaneous murine air pouch inflammatory responses to MSU crystals for their animal model of gout. The subcutaneous air pouches were generated by repeated injection of sterile air into 6-week-old and 8-week-old mice. The researchers found that the AMPKα1 knockout mice had significantly increased MSU crystal-induced leukocyte infiltration in air pouch cavities when compared to wild-type mice (see Figure 1). The MSU crystal-induced inflammation in wild-type mice was inhibited by treatment with the AMPK activator A-769662 (see Figure 2). Western blot analysis of the tissues lining the air pouch confirmed that A-769662 increased phosphorylation of AMPKα. The investigators documented the consequent decreased inflammatory response through IL-1β and CXCL release both in vitro and in vivo.
A-769662 also promoted AMPK-dependent macrophage antiinflammatory M2 polarization in vitro. In particular, A-769662 inhibited MSU crystal-induced phosphorylation of the NF-ҡB p65 subunit in BMDMs. Moreover, treatment with A-769662 inhibited NLRP3 gene expression and activation of caspase-1 and IL-1β.
The latest research suggests that colchicine is an effective treatment for gout because it activates the nutritional biosensor AMPK.
Colchicine Inhibits Inflammatory Response
The team then treated BMDMs that had been stimulated by MSU crystals with a low concentration of colchicine (10 nM). The colchicine dose was selected to mimic the peak plasma concentrations clinically achieved in dosing for gout flare prophylaxis. The investigators observed no significant change in cell viability at this concentration of colchicine. The low dose did, however, promote the phosphorylation of AMPKα as well as macrophage M2 polarization. The polarization was documented by an increase in the ratio of arginase-1 to NOS2 mRNA expression and was observed in the BMDMs from wild-type mice, but not in the BMDMs from AMPKα1 knockout mice. Treatment with colchicine also inhibited the inflammatory response and reduced activation of caspase-1, as well as the release of IL-1β and CXCL1 by wild-type BMDMs, but not knockout BMDMs.
Thus, a decrease in mouse macrophage AMPK activity promotes a state of increased inflammatory responsiveness to MSU crystals. Conversely, AMPK activation and colchicine are able to polarize macrophages in vitro to favor the antiinflammatory M2 population.
Future of Gout Research
The results of the current study open the door to new lines of research in gout pathophysiology and gout treatment. Robert Terkeltaub, MD, professor of medicine at UCSD and a senior investigator on the study, also suggested the possibility that future studies might use an AMPK biomarker in order to better understand the risk of gout flares and elucidate how best to manage the risk of flare. The results of the current study also point to the possibility that additional gout drug therapies might be developed that have an improved ability to increase AMPK activity.
Lara C. Pullen, PhD, is a medical writer based in the Chicago area.
