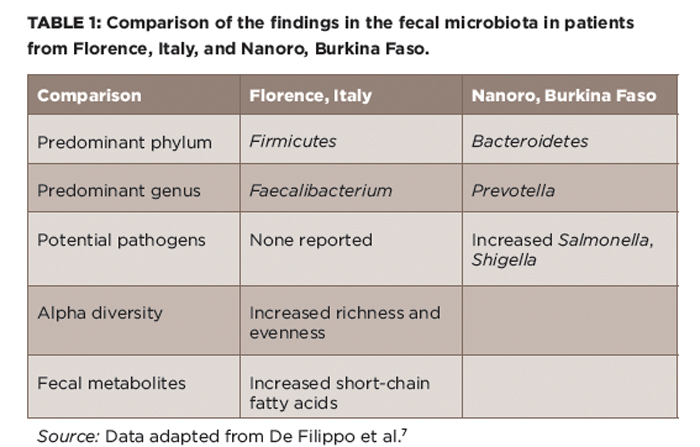
(click for larger image) Table 1: Comparison of the findings in the fecal microbiota in patients from Florence, Italy, and Nanoro, Burkina Faso.
Source: Data adapted from De Filippo et al.7
Or would it? As discussed above, the most likely microbial beneficiary of a diet that resulted in loss of Prevotella would be Bacteroides. Bacteroides fragilis is thought to have anti-inflammatory effects through its polysaccharide A tail.11 However, both rat and mouse models of HLA-B27-associated spondyloarthritis are abrogated in the germ-free state, and in both, the disease comes right back with addition of Bacteroides species.12,13 Further, as noted above, two studies in geographically distinct areas have both identified elevated Bacteroides genus in children with JIA.2,5
Finally, it bears mentioning that P. copri is neither necessary nor sufficient for RA; in the same study by Scher et al, subjects with long-standing RA had similar abundance of P. copri as healthy controls.9 A more recent study of newly diagnosed RA patients conducted in China did not show alterations in the abundance of this organism at diagnosis.14 Thus, the impact of the relative abundance of Prevotella and Bacteroides in the gut microbiota on rheumatic diseases remains unclear.
This is not to indicate that there is no such thing as harmful bacteria. Clearly, such pathogens as Salmonella typhimurium, E. coli 0157:J7, Shigella flexneri and Clostridium difficile are generally less than ideal organisms to dominate one’s microbiota, regardless of their association with inflammatory disease. Conversely, one species, Faecalibacterium prausnitzii, has repeatedly been shown to be deficient in IBD, as well as in ERA/JIA in one study, and is thought to have anti-inflammatory properties in vitro and, likely, in vivo as well.2,4,15 Interestingly, in the Burkina Faso study, the single most common genus in the Florence population was Faecalibacterium, indicating that their population of microbiota may not have been entirely bad.7 It’s possible that with the exception of cases of reactive arthritis triggered by intestinal pathogens such as Salmonella and Shigella, no single organism is either necessary or sufficient for causing most cases of rheumatic disease.
Diversity Matters
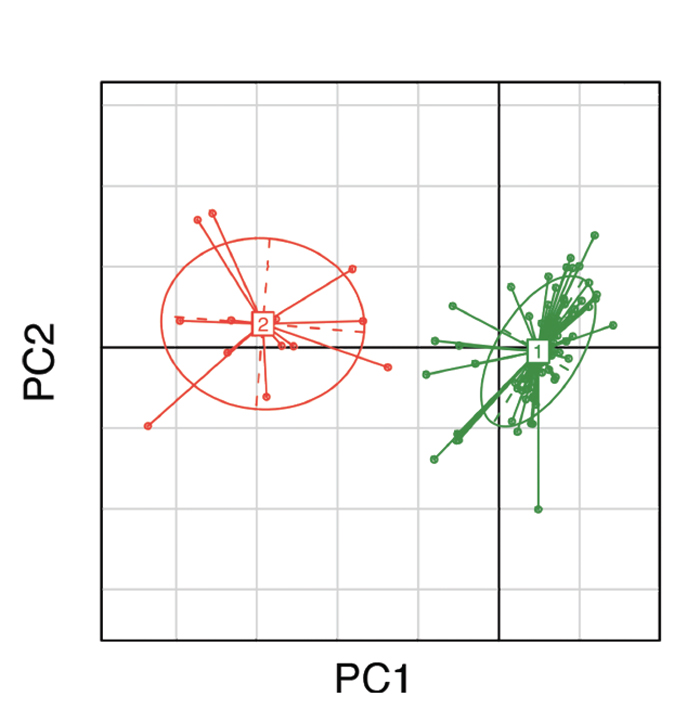
(click for larger image) Figure 1: Clustering of gut microbial bacteria into two distinct enterotypes. Subjects in green represent enterotype 1, which is dominated by Bacteroides; subjects in red represent enterotype 2, which is dominated by Prevotella.10
An alternative possibility is that the significance of the intestinal microbiota may be in the form of its overall community structure. Think of it as a team sport; having a full lineup and a deep bench is surely more useful than a single big-name player. The microbial counterpart to a deep bench is high alpha diversity, which is a measure of the richness, as well as the evenness, of the organisms present in a community. Specifically, richness speaks to the number of different organisms, while evenness speaks to their distribution (see Figure 2).