Monkey Business Images/shutterstock.com
The human intestinal microbiota is home to more than 1,000 bacterial species, containing approximately 3 million genes, many of which code for functions that have the potential to affect human physiology.1 Smaller numbers of organisms are also present in the skin, upper gastrointestinal tract, female reproductive tract and the oro- and nasopharynx. As tools have been developed in the past 10–15 years to query the microbiota, an enormous body of literature addressing the impact of the microbiota on a variety of chronic illnesses has emerged.
One particular condition that has been the subject of recent studies is juvenile idiopathic arthritis (JIA). Our group at the University of Alabama at Birmingham was the first to look at the microbiome in JIA, focusing exclusively on pediatric subjects with enthesitis-related arthritis (ERA, the pediatric version of spondyloarthritis), a condition with known genetic and clinical links to inflammatory bowel disease (IBD).2
We reported decreased fecal abundance of Faecalibacterium prausnitzii, a species with potential anti-inflammatory properties that also appears to be deficient in IBD patients.3,4 Additionally, a subset of patients was identified with a markedly increased abundance of the Bacteroides genus.
The same genus was also shown to be elevated in a Finnish study of children with JIA, mostly with oligo-articular and poly-articular presentations; however, that study did not identify differences in the abundance of F. prausnitzii.5
These studies raise the possibility that some bacteria serve to promote arthritis in children, or even subsets of children, and others play a protective role.
Are There Good & Bad Microbiota?
These studies raise the possibility that there are some beneficial and some not-so-beneficial organisms. This is not a new hypothesis. The concept behind the hygiene hypothesis holds that alterations in the microbiota associated with modernization have resulted in an increased incidence of a variety of immune diseases, such as atopy and IBD.6
Although few would be nostalgic for high perinatal and childhood mortality, the notion that pre-industrialization microbiota may have something to offer us has gained traction. Along those lines, De Filippo et al compared the intestinal microbiota of young children, aged 2–6, in Florence, Italy, with those in a rural village in Burkina Faso, West Africa, a region characterized by an agrarian lifestyle and carbohydrate- and fiber-rich diets.7 There, they found substantial alterations in the intestinal microbiota (see Table 1), including a striking expansion of the Prevotella genus in the Burkina Faso children, with this genus constituting over 50% of the microbiota, while being virtually undetectable in the Florence children. Additional differences included decreases in some potential pathogenic genera and increased microbial diversity observed in the Burkino Faso population. The authors argued that the totality of these findings indicated that the Burkina Faso children likely had a microbiota that could protect them against pathogens, as well as against inflammatory gastrointestinal diseases. It is, however, important to note that there are many differences aside from diet in these two highly diverse populations of children.
Designua/shutterstock.com
It is of interest, then, that two recent studies have come to the opposite conclusion about the single dominant genus present in the Burkina Faso intestines, namely Prevotella. Lukens and colleagues studied the role of the microbiota in a mouse model of chronic recurrent multifocal osteomyelitis (CRMO) mediated by a mutation in the proline-serine-threonine phosphatase-
interacting protein 2 (PSTPIP2) phosphatase.8 Prior to onset of the disease, normal PSTPIP2-deficient mice harbor Prevotella genus at approximately a 10-fold increased abundance compared with wildtype mice, with this abundance increasing at least 20 fold at time of disease. Interestingly, mice fed a high-fat diet (HFD) neither developed this outgrowth of Prevotella, nor did they develop disease, a highly reassuring finding for those of us who fear auto-inflammatory bone disease more than cardiovascular disease. Evidence that the changes in the contents of the microbiota were responsible for the altered disease state included abrogation of the disease with antibiotics, and transfer of the protection mediated by the HFD through fecal transfer from HFD mice to naive mice. Thus, microbiota with high Prevotella genus content may be a contributing risk factor for development of immune disorders.
Another study implicating Prevotella as a potential bad actor in inflammatory disease was a study of newly diagnosed rheumatoid arthritis (RA) patients attending the rheumatology clinic at New York University.9 Scher and colleagues evaluated the intestinal microbiota of 44 new-onset rheumatoid arthritis (NORA) patients and 28 healthy controls (HC), in addition to 26 subjects with chronic RA. High abundance (≥5%) of one particular species of Prevotella, P. copri, was found in the intestines of 33/44 NORA vs. 6/38 HC, a highly statistically significant difference. Having a high abundance of P. copri appeared to compensate in some patients for the absence of the shared epitope HLA alleles that are strong risk factors for RA. Direct evidence that this organism had a pro-inflammatory potential is that colonization of dextran sulfate (DS)-treated C57Bl/6 mice resulted in severe colitis, compared with DS-treated mice gavaged with control media. Moreover, DS-treated animals experienced more weight loss compared with mice gavaged with a species from the Bacteroides genus.
These studies raise the possibility that some bacteria serve to promote arthritis in children, or even subsets of children, & that others play a protective role.
This comparison with Bacteroides is a particularly appropriate choice, because Bacteroides and Prevotella tend to occur in inverse proportions to one another in individual microbiota. This was not only observed by Scher and colleagues, but also in a large study of adults living in the Philadelphia area by Wu et al, in which they correlated intestinal microbiota with dietary intake patterns.10 Consistent with their previous work demonstrating the presence of enterotypes, or predominant intestinal bacteria, they found two basic patterns of fecal microbiota: one dominated by Bacteroides and the other by Prevotella (see Figure 1), with the latter seen in subjects with high starch intake in their diets. This is consistent with the Burkina Faso study, in which the high Prevotella in those children was attributed to their agrarian lifestyle; as well as the Lukens study, which demonstrated that a high-fat diet resulted in loss of Prevotella. Thus, dietary manipulations could be used to reduce intestinal Prevotella, and the studies by Lukens et al and Scher et al suggest that perhaps this would be a good idea.8,9
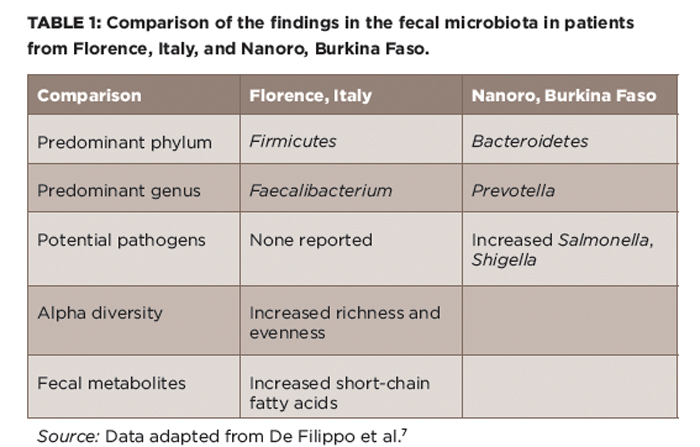
(click for larger image) Table 1: Comparison of the findings in the fecal microbiota in patients from Florence, Italy, and Nanoro, Burkina Faso.
Source: Data adapted from De Filippo et al.7
Or would it? As discussed above, the most likely microbial beneficiary of a diet that resulted in loss of Prevotella would be Bacteroides. Bacteroides fragilis is thought to have anti-inflammatory effects through its polysaccharide A tail.11 However, both rat and mouse models of HLA-B27-associated spondyloarthritis are abrogated in the germ-free state, and in both, the disease comes right back with addition of Bacteroides species.12,13 Further, as noted above, two studies in geographically distinct areas have both identified elevated Bacteroides genus in children with JIA.2,5
Finally, it bears mentioning that P. copri is neither necessary nor sufficient for RA; in the same study by Scher et al, subjects with long-standing RA had similar abundance of P. copri as healthy controls.9 A more recent study of newly diagnosed RA patients conducted in China did not show alterations in the abundance of this organism at diagnosis.14 Thus, the impact of the relative abundance of Prevotella and Bacteroides in the gut microbiota on rheumatic diseases remains unclear.
This is not to indicate that there is no such thing as harmful bacteria. Clearly, such pathogens as Salmonella typhimurium, E. coli 0157:J7, Shigella flexneri and Clostridium difficile are generally less than ideal organisms to dominate one’s microbiota, regardless of their association with inflammatory disease. Conversely, one species, Faecalibacterium prausnitzii, has repeatedly been shown to be deficient in IBD, as well as in ERA/JIA in one study, and is thought to have anti-inflammatory properties in vitro and, likely, in vivo as well.2,4,15 Interestingly, in the Burkina Faso study, the single most common genus in the Florence population was Faecalibacterium, indicating that their population of microbiota may not have been entirely bad.7 It’s possible that with the exception of cases of reactive arthritis triggered by intestinal pathogens such as Salmonella and Shigella, no single organism is either necessary or sufficient for causing most cases of rheumatic disease.
Diversity Matters
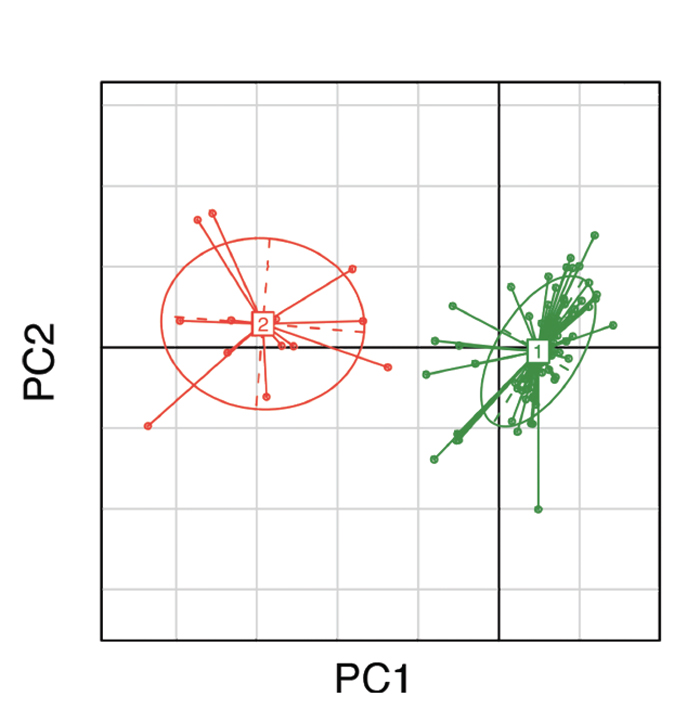
(click for larger image) Figure 1: Clustering of gut microbial bacteria into two distinct enterotypes. Subjects in green represent enterotype 1, which is dominated by Bacteroides; subjects in red represent enterotype 2, which is dominated by Prevotella.10
An alternative possibility is that the significance of the intestinal microbiota may be in the form of its overall community structure. Think of it as a team sport; having a full lineup and a deep bench is surely more useful than a single big-name player. The microbial counterpart to a deep bench is high alpha diversity, which is a measure of the richness, as well as the evenness, of the organisms present in a community. Specifically, richness speaks to the number of different organisms, while evenness speaks to their distribution (see Figure 2).
It turns out that having a highly diverse intestinal microbiota appears to be of benefit in multiple contexts. For example, decreased alpha diversity has been observed in a variety of disorders, including the intestinal microbiota in obesity, irritable bowel syndrome, psoriatic arthritis and IBD; as well as the skin microbiota in psoriasis, vitiligo and atopic dermatitis.16-22 Although cross-sectional studies do not distinguish cause from consequence, two prospective studies have demonstrated that abnormal diversity may be a poor prognostic factor. One of them was an offshoot of a longitudinal study of children in The Environmental Determinants of Diabetes in the Young (TEDDY) database consisting of young children identified as being at increased risk of diabetes based upon their HLA types. Serial stool samples were obtained from asymptomatic high-risk children, with the results showing that alterations in the microbiota—including decreased alpha diversity—were observed prior to the onset of diabetes, such that there was a clear differentiation between these children compared with controls from the TEDDY study who did not develop diabetes.23 Also, a prospective study evaluating the role of the microbiota in 80 subjects undergoing allogeneic stem cell transplantation revealed three-year survival rates of 36%, 60% and 67% among subjects with baseline diversity levels characterized, respectively, as low, intermediate or high.24 To assess the possibility that low diversity was simply a marker for general ill health, the authors adjusted for a variety of predictors, including the following: age, morbidity, the underlying disease and the nature of the conditioning regimen; here again, low-diversity microbial populations emerged as independent predictors of mortality.
Why is having such a diverse microbiota beneficial? There is no definitive answer to this question. However, it may pertain to the functional potential of the microbiota. As noted above, the human intestinal microbiota contains over 3 million genes, many of which can affect various aspects of human physiology, including nutrition, drug metabolism and bile acid recirculation. Decreased diversity at the taxonomic and genetic level may result in decreased diversity at the metabolic level as well.
As an illustration, we performed whole genome sequencing of fecal microbiota plus fecal water metabolomics analyses on a set of children with ERA/JIA and healthy controls. In the ERA/JIA patients, we identified decreased alpha diversity, decreased abundance of genes coding for known metabolic pathways and decreased fecal metabolites.25 The precise association between these findings and disease awaits further exploration, however.
Altering the Microbiota
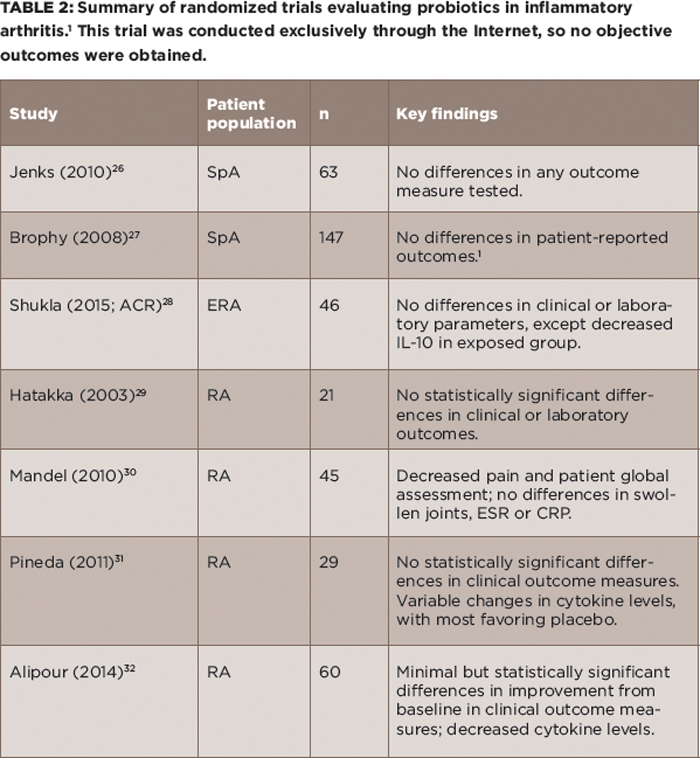
(click for larger image) Table 2: Summary of randomized trials evaluating probiotics in inflammatory arthritis.1 This trial was conducted exclusively through the Internet, so no objective outcomes were obtained.
The holy grail of microbiota-based research is the possibility of effecting changes in the disease state through alterations in the microbiota. As alluded to earlier, this can be achieved in mouse models of inflammatory disease; however, in humans the scope of possible changes is narrowed and the changes generally would have to be initiated after development of disease. With these caveats in mind, there are four basic ways to alter the microbiota (probiotics, antibiotics, diet and fecal transplant), all of which have been tested in humans to varying degrees.
Probiotics: If there is an imbalance in the microbiota, then the instinctual response is to ingest new ones. Although this possibility is appealing conceptually, it has not panned out well as a therapeutic option for rheumatic disease. Randomized trials of probiotics in adult and pediatric SpA both failed to show any effect, and likewise, studies in RA have shown minimal if any benefit (see Table 2).26-32
There are several reasons why this might be the case. For one, none of these trials were predicated upon an understanding of which microbiota may have been deficient in the patient population. Thus, concluding that the microbiota are irrelevant to disease on the basis of the negative probiotic trials would be akin to giving up on drug therapy.
Second, to the extent that one of the predisposing factors in inflammatory arthritis is decreased diversity, taking large numbers each day of a single or small set of organisms whose a priori abundance has not even been established may not address the underlying issue.
Finally, in the context of several trillion organisms firmly embedded in their intestinal niches, it is not clear to what extent the introduction of new organisms will even alter the microbiota because this has not been assessed in these studies. Think of it as trying to board a rush-hour subway that is already bursting at the seams.
That said, there have been successes with probiotic therapy. They are widely used in the management of ulcerative colitis; these patients likely have available niches due to the inflammatory process, as well as extensive antibiotic use.33 Additionally, a recent study using the same TEDDY database mentioned above showed probiotics to be effective in at-risk children if, and only if, administered prior to 27 days of life.34 The microbiota of neonates is in a high state of flux, so effectiveness in this age group does not necessarily translate to success in older children and adults. Nevertheless, more sophisticated approaches to altering the microbiota with probiotics will likely continue to be assessed in treating rheumatic disease.
Antibiotics: Another shotgun approach to altering the gut microbiota in rheumatic disease is the use of antibiotics. However, antibiotics may be a double-edged sword with respect to inflammatory arthritis. Several recent studies evaluating large European databases have shown that early use of antibiotics may be a risk factor for subsequent development of pediatric IBD and JIA.35-38 However, although antibiotics don’t appear to be effective in SpA, studies of their effect in adult RA patients have demonstrated success.39,40
The microbial counterpart to a deep bench is high alpha diversity, which is a measure of the richness, as well as the evenness, of the organisms present in a community. Specifically, richness speaks to the number of different organisms, while evenness speaks to their distribution. Thus, a balanced & diverse microbiota may be optimal for maintaining health.
It’s hard to imagine that a disease potentially caused by antibiotics (e.g., JIA) could then be treated by antibiotics. Moreover, the risks of long-term antibiotic use, such as infection with C. difficile, also discourage their use. Further, antibiotics are fairly indiscriminate in their effect on the microbiota, wiping out both beneficial and harmful organisms and undoubtedly adversely affecting diversity. There has been very little interest in their use in the biologic era, and at present, it is difficult to propose introducing them as part of the therapeutic armamentarium for children with JIA. However, the positive results of some of the studies in using antibiotics to treat rheumatic disorders speak to the potential of therapeutic alterations of the microbiome.
Diet: Perhaps, a safer approach to altering gut microbiota involves dietary changes. That dietary changes can rapidly effect alterations in the microbiome is well established. For example, the study by Wu and colleagues also involved an interventional arm, in which 10 subjects were randomized to either a low-fat or a high-fat diet for 10 days, with substantial changes seen in some subjects after just one day.10 In light of the typical American diet, the changes appeared to be more pronounced among those randomized to the low-fat diet compared with those randomized to the high-fat diet.
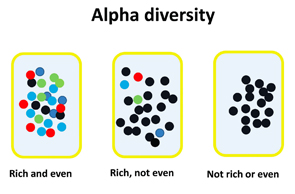
Figure 2: Illustration of the concepts of richness and evenness within alpha diversity. Richness refers to the variety of different organisms; evenness refers to the distribution of the organisms within the population.
An extreme form of dietary change consists of exclusive enteral nutrition (EEN), which appears to be comparable to corticosteroids as induction therapy in pediatric IBD.41 On the other hand, dietary interventions in RA have not been particularly effective.42 There are no data in children, aside from a case report of EEN benefit in a child with refractory polyarticular JIA.43 It must be emphasized that just because a particular diet may alter the microbiota, it does not follow that this alteration is beneficial with respect to any particular disease process. In addition, many diets may be very difficult to sustain. The challenge will be in identifying a diet that promotes beneficial changes in the microbiota, provides appropriate balanced nutrition and is acceptable to families for the long term.
Fecal microbial transplant: The latest approach to altering the human gut microbiome is fecal microbial transplant (FMT). Initially introduced as therapy for recurrent C. difficile infection, interest has emerged in the use of FMT as therapy for IBD, with mixed results to date.44,45 Evidently, anyone with a blender and a funnel can do this at home, although one might recommend limiting this to academic centers with proper screening procedures and procedural expertise (http://thepowerofpoop.com/epatients/fecal-transplant-instructions). The safety and utility of FMT for treating the vast majority of rheumatic conditions are currently unknown.
Concluding Remarks
Information about the contents and potential role of the microbiota in inflammatory diseases is rapidly advancing. Animal studies provide compelling evidence for an important role for the microbiota in the pathogenesis of inflammatory diseases. Although most human studies have been associative in nature, some studies have shown prognostic capacity of altered microbial diversity, and measures that alter the microbiota may have a therapeutic effect as well. Whether the next decade will see the emergence of microbiota-based therapies remains to be seen.
 Matthew Stoll, MD, PhD, MSCS, is an associate professor of pediatrics in the Division of Pediatric Rheumatology at the University of Alabama at Birmingham. His clinical and research interests are in pediatric spondyloarthritis and in the role of the microbiome in the pathogenesis of arthritis.
Matthew Stoll, MD, PhD, MSCS, is an associate professor of pediatrics in the Division of Pediatric Rheumatology at the University of Alabama at Birmingham. His clinical and research interests are in pediatric spondyloarthritis and in the role of the microbiome in the pathogenesis of arthritis.
References
- Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010 Mar 4;464:59–65.
- Stoll ML, Kumar R, Morrow CD, et al. Altered microbiota associated with abnormal humoral immune responses to commensal organisms in enthesitis-related arthritis. Arthritis Res Ther. 2014 Nov 30;16(6):486.
- Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008 Oct 28;105(43):16731–16736.
- Cao Y, Shen J, Ran ZH. Association between Faecalibacterium prausnitzii reduction and inflammatory bowel disease: A meta-analysis and systematic review of the literature. Gastroenterol Res Pract. 2014;2014:872725.
- Tejesvi MV, Arvonen M, Kangas SM, et al. Faecal microbiome in new-onset juvenile idiopathic arthritis. Eur J Clin Microbiol Infect Dis. 2016 Mar;35(3):363–370.
- Rook GA. Hygiene hypothesis and autoimmune diseases. Clin Rev Allergy Immunol. 2012 Feb;42(1):5–15.
- De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010 Aug 17;107(33):14691–14696.
- Lukens JR, Gurung P, Vogel P, et al. Dietary modulation of the microbiome affects autoinflammatory disease. Nature. 2014 Dec 11;516(7530):246–249.
- Scher JU, Sczesnak A, Longman RS, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013 Nov 5;2:e01202.
- Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011 Oct 7;334(6052):105–108.
- Round JL, Lee SM, Li J, et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011 May 20;332:974–977.
- Rath HC, Herfarth HH, Ikeda JS, et al. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest. 1996 Aug 15;98:945–953.
- Sinkorova Z, Capkova J, Niederlova J, et al. Commensal intestinal bacterial strains trigger ankylosing enthesopathy of the ankle in inbred B10.BR (H-2(k)) male mice. Hum Immunol. 2008 Dec;69(12):845–850.
- Zhang X, Zhang D, Jia H, et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med. 2015 Aug;21(8):895–905.
- Stoll ML. Gut microbes, immunity, and spondyloarthritis. Clinical Immunol. 2015 Aug;159(2):134–142.
- Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009 Jan 22;457:480–484.
- Durban A, Abellan JJ, Jimenez-Hernandez N, et al. Structural alterations of faecal and mucosa-associated bacterial communities in irritable bowel syndrome. Environ Microbiol Rep. 2012 Apr;4(2):242–247.
- Scher JU, Ubeda C, Artacho A, et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol. 2015 Jan;67(1):128–139.
- Michail S, Durbin M, Turner D, et al. Alterations in the gut microbiome of children with severe ulcerative colitis. Inflamm Bowel Dis. 2012 Oct;18(10):1799–1808.
- Alekseyenko AV, Perez-Perez GI, De Souza A, et al. Community differentiation of the cutaneous microbiota in psoriasis. Microbiome. 2013 Dec 23;1(1):31.
- Ganju P, Nagpal S, Mohammed MH, et al. Microbial community profiling shows dysbiosis in the lesional skin of Vitiligo subjects. Sci Rep. 2016 Jan 13;6:18761.
- Nylund L, Nermes M, Isolauri E, et al. Severity of atopic disease inversely correlates with intestinal microbiota diversity and butyrate-producing bacteria. Allergy. 2015 Feb;70(2):241–244.
- Giongo A, Gano KA, Crabb DB, et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 2011 Jan;5(1):82–91.
- Taur Y, Jenq RR, Perales MA, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014 Aug 14;124(7):1174–1182.
- Stoll ML, Wilson L, Barnes S, et al. Multi-omics study of gut microbiota in enthesitis-related arthritis identify diminished microbial diversity and altered typtophan metabolism as potential factors in disease pathogenesis [abstract 2820]. Arthritis Rheum. 2015 Sep 29;67(suppl 10).
- Jenks K, Stebbings S, Burton J, et ak. Probiotic therapy for the treatment of spondyloarthritis: A randomized controlled trial. J Rheumatol. 2010 Oct;37(10):2118–2125.
- Brophy S, Burrows CL, Brooks C, et al. Internet-based randomised controlled trials for the evaluation of complementary and alternative medicines: Probiotics in spondyloarthropathy. BMC Musculoskelet Disord. 2008 Jan 11;9:4.
- Shukla A, Gaur P, Aggarwal A. Double blind placebo controlled randomized trial of probiotics in enthesitis-related-arthritis category of JIA: Effect on clinical and immunological parameters [abstract 957]. Arthritis Rheum. 2015 Sep 29;67(suppl 10).
- Hatakka K, Martio J, Korpela M, et al. Effects of probiotic therapy on the activity and activation of mild rheumatoid arthritis—A pilot study. Scand J Rheumatol. 2003;32(4):211–215.
- Mandel DR, Eichas K, Holmes J. Bacillus coagulans: A viable adjunct therapy for relieving symptoms of rheumatoid arthritis according to a randomized, controlled trial. BMC Complement Altern Med. 2010 Jan 12;10:1.
- Pineda Mde L, Thompson SF, Summers K, et al. A randomized, double-blinded, placebo-controlled pilot study of probiotics in active rheumatoid arthritis. Med Sci Monit. 2011 Jun;17(6):CR347–CR354.
- Alipour B, Homayouni-Rad A, Vaghef-Mehrabany E, et al. Effects of Lactobacillus casei supplementation on disease activity and inflammatory cytokines in rheumatoid arthritis patients: A randomized double-blind clinical trial. Int J Rheum Dis. 2014 Jun;17(5):519–527.
- Chibbar R, Dieleman LA. Probiotics in the management of ulcerative colitis. J Clin Gastroenterol. 2015 Nov–Dec;49(Suppl 1):S50–S55.
- Uusitalo U, Liu X, Yang J, et al. Association of early exposure of probiotics and islet autoimmunity in the TEDDY study. JAMA Pediatr. 2016 Jan 1;170(1):20–28.
- Kronman MP, Zaoutis TE, Haynes K, et al. Antibiotic exposure and IBD development among children: A population-based cohort study. Pediatrics. 2012 Oct;130(4):e794–e803.
- Virta L, Auvinen A, Helenius H, et al. Association of repeated exposure to antibiotics with the development of pediatric Crohn’s disease—A nationwide, register-based Finnish case-control study. Am J Epidemiol. 2012 Apr 15;175(8):775–784.
- Arvonen M, Virta LJ, Pokka T, et al. Repeated exposure to antibiotics in infancy: A predisposing factor for juvenile idiopathic arthritis or a sign of this group’s greater susceptibility to infections? J Rheumatol. 2015 Mar;42(3):521–526.
- Horton DB, Scott FI, Haynes K, et al. Antibiotic exposure and juvenile idiopathic arthritis: A case-control study. Pediatrics. 2015 Aug;136(2):e333–e343.
- Barber CE, Kim J, Inman RD, et al. Antibiotics for treatment of reactive arthritis: A systematic review and metaanalysis. J Rheumatol. 2013 Jun;40(6):916–928.
- Saviola G, Abdi-Ali L, Campostrini L, et al. Clarithromycin in rheumatoid arthritis: The addition to methotrexate and low-dose methylprednisolone induces a significant additive value—A 24-month single-blind pilot study. Rheumatol Int. 2013;33(11):2833–2838.
- Heuschkel RB, Menache CC, Megerian JT, Baird AE. Enteral nutrition and corticosteroids in the treatment of acute Crohn’s disease in children. J Pediatr Gastroenterol Nutr. 2000 Jul;31(1):8–15.
- Hagen KB, Byfuglien MG, Falzon L, et al. Dietary interventions for rheumatoid arthritis. Cochrane Database Syst Rev. 2009 Jan 21;(1):CD006400.
- Berntson L. Anti-inflammatory effect by exclusive enteral nutrition (EEN) in a patient with juvenile idiopathic arthritis (JIA): Brief report. Clin Rheumatol. 2014 Aug;33(8): 1173–1175.
- Suskind DL, Brittnacher MJ, Wahbeh G, et al. Fecal microbial transplant effect on clinical outcomes and fecal microbiome in active Crohn’s disease. Inflamm Bowel Dis. 2015 Mar;21(3):556–563.
- Suskind DL, Singh N, Nielson H, Wahbeh G. Fecal microbial transplant via nasogastric tube for active pediatric ulcerative colitis. J Pediatr Gastroenterol Nutr. 2015 Jan;60(1):27–29.