Alila Medical Media / shutterstock.com
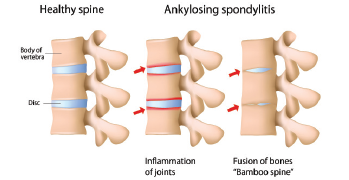
What factors drive inflammation and progressive disease in ankylosing spondylitis (AS)? The answers have long eluded rheumatologists. Although 90% of patients with AS test positive for the HLA-B27 gene, pieces remain missing in our understanding of this chronic, inflammatory disease, which often leads to pain, spinal fusion and, in about half of patients, gut involvement, such as a bowel disorder.1,2
Past research has identified cells that drive inflammation and new bone formation in spondyloarthritis (SpA) studies in mice, but not in humans, says Nigil Haroon, MD, PhD, Krembil Research Institute, University of Toronto in Ontario, Canada.3
“The difficulty in assessing tissue is a major factor affecting our ability to study the pathogenesis of AS. Back pain is a common symptom seen in the general population, but only a small portion of these patients have AS as the cause of their pain,” he says. “There is an average delay of eight years from symptom onset to AS diagnosis, so we have limited ability to study the preclinical and early stages of the disease. Animal models available for SpA [don’t provide] a perfect representation of human disease.”
In a new paper in the September issue of Arthritis & Rheumatology, Dr. Haroon and his colleagues reveal the likely culprit: macrophage migration inhibitory factor (MIF).4 According to their study, “Macrophage Migration Inhibitory Factor Induces Inflammation and Predicts Spinal Progression in Ankylosing Spondylitis,” this potent cytokine drives the two major disease processes in AS and could be both a new target for drug development and a potential biomarker to identify AS patients with more aggressive disease, says Dr. Haroon.
“If proved in preclinical trials, MIF would be an ideal therapeutic target [for] clinical trials in AS,” he says. “MIF appears to be an independent predictor of spinal fusion, and in vitro and in vivo, it can directly drive osteoblast activation and mineralization.”
Gut–Spine Connections
The study aimed to learn more about AS disease progression and a long-suspected gut–joint link, says Dr. Haroon. “[We have] several clues to this link. Just to name a few, 10% of AS patients have clinical inflammatory bowel disease, [but] 60–70% have microscopic colitis. Mucosa-associated invariant T cells [MAIT cells] and other gut-derived cells have been implicated in arthritis,” he says, adding that MIF also plays a key role in the development of chronic colitis in mice.5 MIF, which helps regulate innate immunity, may also play a role in other inflammatory autoimmune diseases, as well as sepsis.6
