Image Credit: Bork/shutterstock.com
Musculoskeletal (MSK) ultrasound is a valuable imaging modality for the practicing rheumatologist and provides an efficient tool with high diagnostic value in the evaluation of patients with musculoskeletal complaints. The use of MSK ultrasound has evolved in the U.S. due to the emergence of less-expensive, portable ultrasound units, which provide high-quality gray-scale and power Doppler signals. The American College of Rheumatology (ACR) has provided standardized training courses, as well a certification program, for the practicing rheumatologist, and such training is also available in many fellowship programs.1
The diagnosis of rheumatoid arthritis (RA) can be challenging in certain clinical settings because crystalline arthropathies can be close mimics of RA. For example, palindromic RA can present with articular symptoms, which can resemble a crystalline process based on historical and clinical features.
The following cases demonstrate the usefulness of ultrasound in providing clues to the underlying diagnosis and highlight a case in which this imaging modality was helpful in assessing disease activity in an established RA patient, which altered the patient management.
Case 1
A 57-year-old male with a past medical history of RA was referred to rheumatology by his primary care physician (PCP). The patient had been diagnosed with RA four years earlier, with manifestations of bilateral hand symptoms along with high titer rheumatoid factor (RF) and anti-cyclic citrullinated peptide (CCP) antibodies. He was initially treated by a rheumatologist and had started oral methotrexate, but discontinued it after a few months due to his concerns of long-term drug toxicity. He did not follow up with his rheumatologist and remained off treatment for more than three years. During this time period, he tried numerous herbal products, along with acupuncture, with negligible improvement and was encouraged to follow up with rheumatology by his PCP.
MSK ultrasound can enhance diagnostic certainty for the diagnosis of RA because subtle clinical exam signs of synovitis or tenosynovitis can be quite obvious on ultrasound examination.
At the time of follow-up, his symptoms included mild pain and morning stiffness in his feet and hands (30 minutes’ duration) along with
(click for larger image)
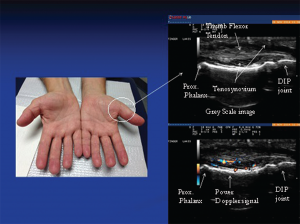
Image 1: The photo (left) reveals subtle soft tissue swelling of the proximal portion of left thumb (arrow) corresponding to teno-synovial hypertrophy seen on this longitudinal gray-scale image of the proximal portion of the thumb. Doppler ultrasound of the same area (right): Power Doppler signals indicate inflammatory (high vascularity) signal of the surrounding tenosynovium deep to the flexor tendons reflective of tenosynovitis.
nocturnal hand paresthesias. His physical examination was significant for subtle soft tissue swelling over the left first metacarpophalangeal (MCP) joint with an impression of fullness over the volar aspect of the left wrist upon inspection (see Image 1). On palpation of the involved joints, he had minimal tenderness. Ultrasound of the volar wrist within the carpal tunnel revealed tenosynovial hypertrophy along with strikingly positive power Doppler signals on both longitudinal and transverse views consistent with active tenosynovitis (see Image 1, above).
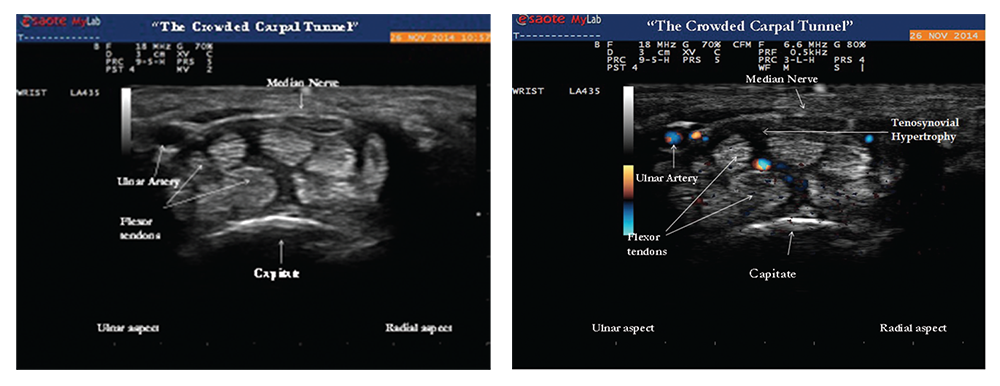
The median nerve was normal in cross‑sectional area (9 mm2) and appeared hypoechoic, but was significantly enlarged compared with a more proximal portion of the median nerve (6 mm2). A size ratio comparison of distal to proximal median nerve (9 mm2/6 mm2) was calculated to be 1.5 and ratios greater than 1.4 are suggestive of median nerve entrapment.2 The ultrasound findings of carpal tunnel syndrome are also defined by enlargement of the median nerve by cross-sectional area of measurements equal to or greater than 12 mm2. In the clinical context of hand paresthesias, the ultrasound images of distal median nerve enlargement suggest carpal tunnel syndrome and this was confirmed by electromyographic nerve conduction studies (see Images 2 and 3).
(click for larger image)
Images 2 & 3: Gray-scale image (left) of the transverse view of the wrist showing tenosynovial hypertrophy within the proximal portion of the carpal tunnel. The hazy dark areas between the flexor tendons represent tenosynovial proliferation with effect of “crowding” the carpal tunnel by increasing perineural median nerve pressure. Doppler ultrasound (right) of the same area reveals positive power Doppler signals between the flexor tendons, an abnormal finding consistent with tenosynovitis.
The patient was nihilistic and reluctant to take medications because he was still able to function with his symptoms; however, ultrasound provided objective evidence that convinced him to start disease-modifying anti-rheumatic drug (DMARD) therapy. The patient agreed to start etanercept therapy and understood the importance of subsequent follow-up care for proper treatment of his rheumatic disease.
Per the 2010 rheumatoid arthritis classification criteria, joint involvement, serology, acute-phase reactants and duration of symptoms are used for the diagnosis of RA. Patients with typical patterns of joint involvement (>10 joints with at least one small joint) and duration of symptoms for more than six weeks meet the clinical criteria for RA.3 The exclusion of RA mimics, such as viral and crystalline arthropathies, is critical to an accurate diagnosis.
(click for larger image)
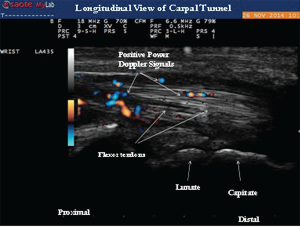
Image 4: Longitudinal view of the carpal tunnel demonstrating the positive power Doppler signals between the flexor tendons.
In early RA, acute-phase reactants, serologies and plain radiographs can be normal, making the history and physical examination of primary importance to the diagnosis. MSK ultrasound can enhance diagnostic certainty for the diagnosis of RA because subtle clinical exam signs of synovitis or tenosynovitis can be quite obvious on ultrasound examination. In addition, MSK ultrasound can provide reliable imaging findings for the presence of crystalline arthropathies, which can be helpful in identifying the crystalline mimics of RA.
EULAR recommendations for use of imaging of joints in clinical management of patients with RA support the use of ultrasound at various disease stages. Ultrasound can be used to diagnose RA when there is diagnostic uncertainty, and this imaging modality can accurately detect joint inflammation (superior to clinical examination). Ultrasound should be considered in patients with early RA if conventional radiography is normal and exam findings are questionable for active tenosynovitis or synovitis. It’s useful to monitor disease activity and progression, to predict further joint damage as a prognostic indicator, and to predict treatment response. Moreover, ultrasound can detect inflammation during clinical remission and predict subsequent joint damage by assessing persistent inflammation as defined by positive power Doppler activity.4 In addition, as in our patient, ultrasound imaging can provide objective evidence of active synovitis, which can be of value when educating patients on the importance of initiating DMARD therapy.
(click for larger image)
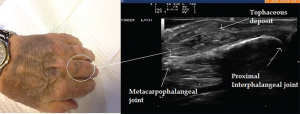
Image 5: Ultrasound image of inhomogeneous hyperechoic signals in the soft tissue appearance. The area was suggestive of tophi and aspirated under ultrasound guidance with confirmation.
Case 2
A 73-year-old male with a past medical history significant for renal transplant secondary to polycystic kidney disease presented to the rheumatology office with complaints of soreness and swelling in his left third proximal interphalangeal joint (PIP) for a duration of two months. His immunosuppressive regimen included tacrolimus and prednisone 5 mg daily. The patient also had pain and swelling associated with stiffness in both ankles.
His chronic joint complaints were suggestive of either RA or a microcrystalline process. On examination he had soft tissue swelling of the third PIP joint with adjacent fullness of the proximal phalanx. No visible cutaneous tophi were appreciated on exam.
The EULAR recommendations for CPP deposition suggest that ultrasound is more sensitive & specific than X-rays for peripheral joints.
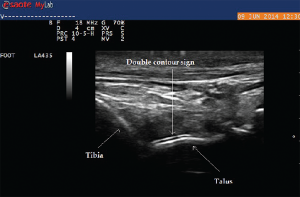
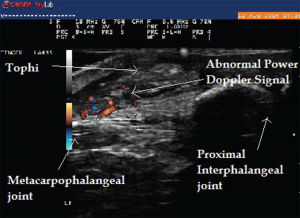
Ultrasound imaging of the third PIP revealed peri-articular swelling with hyperechoic signals in a focal aggregate associated with surrounding positive power Doppler signals (see Images 5 & 6). The area was aspirated under ultrasound guidance revealing tophaceous material (see Image 5 confirmed with a polarizing microscope examination. The patient was maintained on low-dose steroids and then transitioned to the uricosuric agent febuxostat with gradual improvement of his symptoms. His uric acid level on his initial visit was 8.6 mg/dL, which improved to 4.5 mg/dL. Of note, the patient was not on cyclosporine, which increases the propensity to development of gout in transplant patients. He was on low-dose prednisone for his renal transplant, which may have masked the typical presentation of gout. Of interest, the ultrasound of the intermittently symptomatic right ankle demonstrated double contour sign of the tibiotalar joint (see Image 7).
(click for larger image)
Image 6: Abnormal power Doppler signals suggestive of inflammation and high vascularity in the same patient.
Several findings on the musculoskeletal ultrasound are characteristic of gout. Hyperechoic spots less than 1 mm in size within anechoic synovial fluid can result in a “snowstorm appearance,” with movement of these deposits within the synovial fluid. Tophi deposits have a varied appearance and can be seen as a circumscribed, inhomogeneous, hyperechoic and/or hypoechoic aggregation, which may be surrounded by a small anechoic rim.5 Hard tophi can generate well-defined hyperechoic deposits with posterior acoustic shadows, whereas soft tophi can appear hypoechoic with hyperechoic stippled aggregates or bright dotted foci without a posterior acoustic shadow.6
Urate deposition within the synovial tissue can also generate hyperechoic aggregates with a cloudy surrounding rim of hypoechoic signal, with the appearance of wet clumps of sugar. The most specific and helpful finding is the double contour sign, which is a hyperechoic band over the superficial surface of the cartilage reflecting urate deposition on the cartilage surface. It can be differentiated from the interface sign because the double contour sign will have a signal thickness and echogenicity similar to subchondral bone signal, which is unchanged with insonation angle.6 In addition, ultrasound can detect minimal amounts of intra-articular synovial fluid appearing as a compressible anechoic collection and can be used for needle guidance, particularly for joints more challenging to aspirate blindly (i.e., ankle, elbow or the root joints, such as the shoulder or hip).
(click for larger image)
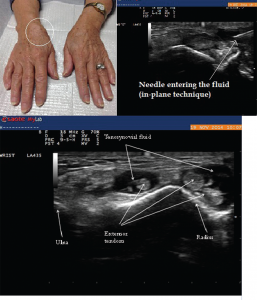
Image 8: Right wrist swelling (left) with focal fusiform swelling over the dorsum of the wrist. To the right, you see the needle entering the tenosynovial fluid (in-plane technique). Bottom: The longitudinal view along the course of extensor pollicis longus taken just proximal to Lister’s tubercle, demonstrating compressible fluid (anechoic or dark area), without tenosynovial hypertrophy. Fluid was aspirated using ultrasound guidance, which demonstrated inflammatory fluid (leukocytes=5545/High Power Field) with calcium pyrophosphate crystals on microscopic exam.
Multiple tiny hyperechoic spots in anechoic synovial fluid are thought to be the earliest ultrasound evidence of crystal deposition.7 In the Study for Updated Gout ClAssification CRiteria (SUGAR), funded by the ACR and EULAR, the double contour sign on ultrasound is one of the 10 key discriminating features that have been identified for further study for new gout classification criteria. Ultrasound findings, along with degree of uricemia, add discriminating value and will significantly contribute to more accurate classification criteria for gout.8 In addition, the presence of peri-articular and intra-articular power Doppler signals, synovial hypertrophy, effusion and soft tissue edema are all indicative of synovitis due to acute crystalline inflammation.
In our patient, MSK ultrasound was helpful in identifying a peri-articular tophaceous deposit with surrounding inflammation that was difficult to appreciate on exam. Needle guidance also increased the diagnostic yield of our needle aspiration.
Case 3
A 79-year-old female presented to the rheumatology office with complaints of pain in her right wrist. She described the pain as dull achy pain, associated with one hour of stiffness that interfered with her daily tasks. She also had mild pain at rest. These symptoms had been present for seven months. Her physical examination was significant for diffuse joint tissue swelling over the dorsum of the wrist, with tenderness over the radial aspect of the joint (see Image 8).
X-rays of her wrists showed osteoarthritic changes to the first carpometacarpal (CMC) and trapezium-scaphoid joint without evidence of chondrocalcinosis. Ultrasound of her wrists showed prominent tenosynovial effusion, inhomogeneous synovium and abnormal power Doppler signals in the wrist joint.
Ultrasound can detect minimal amounts of intra-articular synovial fluid appearing as a compressible anechoic collection & can be used for needle guidance.
Aspiration of the effusion using ultrasound guidance revealed turbid synovial fluid with a leukocyte count of 5,200 per microliter with intracellular calcium pyrophosphate crystals. She had an incomplete response to colchicine, but did respond well to local ultrasound-guided injection to the tenosynovial sheath and radial carpal joint.
(click for larger image)
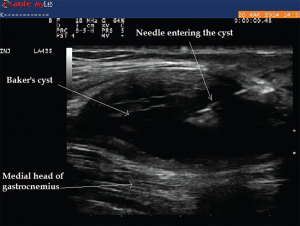
Image 9: The longitudinal view of Baker’s cyst. The medial gastrocnemius muscle is not seen on this view. The needle tip during aspiration using an in-plane technique can be visualized within the cyst.
Ultrasound findings of calcium pyrophosphate crystal deposition (CPPD) disease usually are seen within the hyaline cartilage and appear as hyperechoic spots that can be isolated, or form confluent spots that don’t generate an acoustic shadow.9 Similarly, calcium pyrophosphate dehydrate (CPP) deposition within fibro-cartilaginous structures, such as the meniscus, can appear as hyperechoic rounded or amorphous deposits. The medial meniscus of the knee is the most commonly affected cartilage among these patients.10
Presence of hyperechoic aggregates may be indicative of crystals; ultrasound-guided aspiration of these crystals can increase the sensitivity of diagnosis of the disease. Radiographs of multiple joints to prove the diagnosis can be cumbersome and exposes the patient to unnecessary radiation. The EULAR recommendations for CPP deposition suggest ultrasound is more sensitive and specific than X-rays for peripheral joints.11
Case 4
A 75-year-old female with a history of seronegative RA on low-dose prednisone, hydroxychloroquine and leflunomide presented to the rheumatology office with complaints of posterior knee pain and swelling, which had gradually worsened in the past month. She was compliant with her medications, and there was no recent change in her medications. Ultrasound of her knee demonstrated a large Baker’s cyst, and ultrasound-guided aspiration of the fluid demonstrated class I fluid. Typically, on a transverse view, boundaries of Baker’s cyst are formed by semimembranosus and semitendinosus of the medial aspect and medial gastrocnemius on the lateral aspect. Ultrasound allowed for diagnosis and therapeutic intervention during the same visit without the need to refer to radiology (see Images 9 and 10).
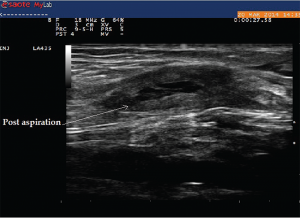
(click for larger image)
Image 10: This image was taken after aspiration of the fluid from the cyst.
Conclusion
MSK ultrasound is an imaging modality with practical application for the clinical rheumatologist. A growing literature supports its reliability in diagnosing early RA and assessing disease activity in established patients in addition to improving accuracy by excluding RA mimics. The added benefit of needle guidance can increase the diagnostic yield of arthrocentesis, which may obviate the need for radiology referral for root joint aspirations (hip and shoulder) or deep bursal aspirations.
Further study of this imaging modality and recognition of its application for the clinician will enhance treatment of rheumatic disease patients.
Khushboo Sheth, MBBS, is a resident in Internal Medicine at the University of Connecticut in Farmington, Conn.
Christopher Scola, MD, is chief of the Rheumatology Division at Hartford Hospital and assistant clinical professor of medicine at the University of Connecticut School of Medicine, Hartford, Conn.
References
- American College of Rheumatology. Education and Careers.
- Hobson-Webb LD, Massey JM, Juel VC, Sanders DB. The ultrasonographic wrist-to-forearm median nerve area ratio in carpal tunnel syndrome. Clin Neurophysiol. 2008 Jun;119(6):1353–1357.
- Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010 Sep;62(9):2569–2581.
- Colebatch AN, Edwards CJ, Ostergaard M, et al. EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann Rheum Dis. 2013 Jun;72(6):804–814.
- Gutierrez M, Schmidt WA, Thiele RG, et al. International consensus for ultrasound lesions in gout: Results of delphi process and Web-reliability exercise. Rheumatology (Oxford). 2015 May 13; pii.
- Perez-Ruiz F, Dalbeth N, Urresola A, et al. Imaging of gout: Findings and utility. Arthritis Res Ther. 2009;11(3):232.
- Grassi W, Okano T, Filippucci E. Use of ultrasound for diagnosis and monitoring of outcomes in crystal arthropathies. Curr Opin Rheumatol. 2015 Mar;27(2):147–155.
- Taylor WJ, Fransen J, Jansen TL, et al. Study for updated gout classification criteria (SUGAR): Identification of features to classify gout. Arthritis Care Res (Hoboken). 2015 Mar 16.
- Wakefield RJ, D’Agostino MA. Essential Applications of Musculoskeletal Ultrasound in Rheumatology: Expert Consult, premium ed. Elsevier Health Sciences. 2010.
- Frediani B, Filippou G, Falsetti P, et al. Diagnosis of calcium pyrophosphate dihydrate crystal deposition disease: Ultrasonographic criteria proposed. Ann Rheum Dis. 2005 Apr;64(4):638–640.
- Zhang W, Doherty M, Bardin T, et al. European League Against Rheumatism recommendations for calcium pyrophosphate deposition. part I: Terminology and diagnosis. Ann Rheum Dis. 2011 Apr;70(4):563–570.