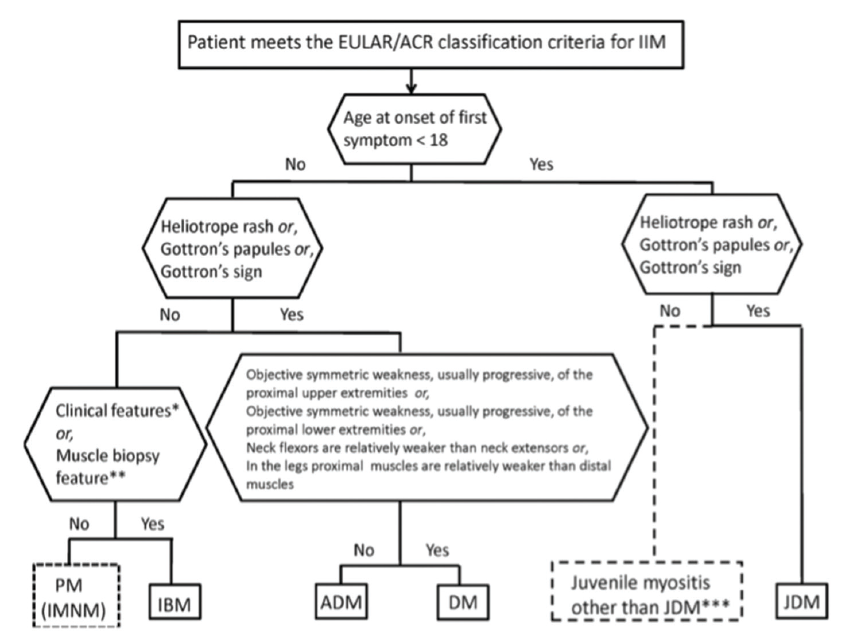
(click for larger image) Figure 1: Classification Tree for Subgroups of Idiopathic Inflammatory Myopathies4
Novel Autoantibodies
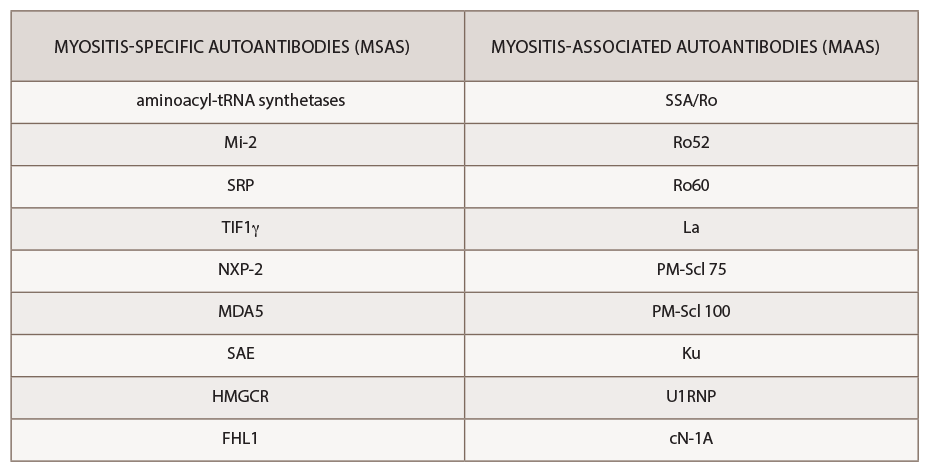
The past decade has seen an increasing recognition of novel autoantibodies identified in types of inflammatory myopathy. Autoantibodies divide into two classifications: myositis-specific antibodies (MSAs), which are almost exclusively present in IIM, and myositis-associated antibodies (MAAs), which are present in other systemic autoimmune diseases (see Table 3). Myositis-specific antibodies can be tested by ordering myositis antibody panels available commercially. Based on published literature, MSAs or disease-specific antibodies can be identified in over 70% of patients with IIM.5
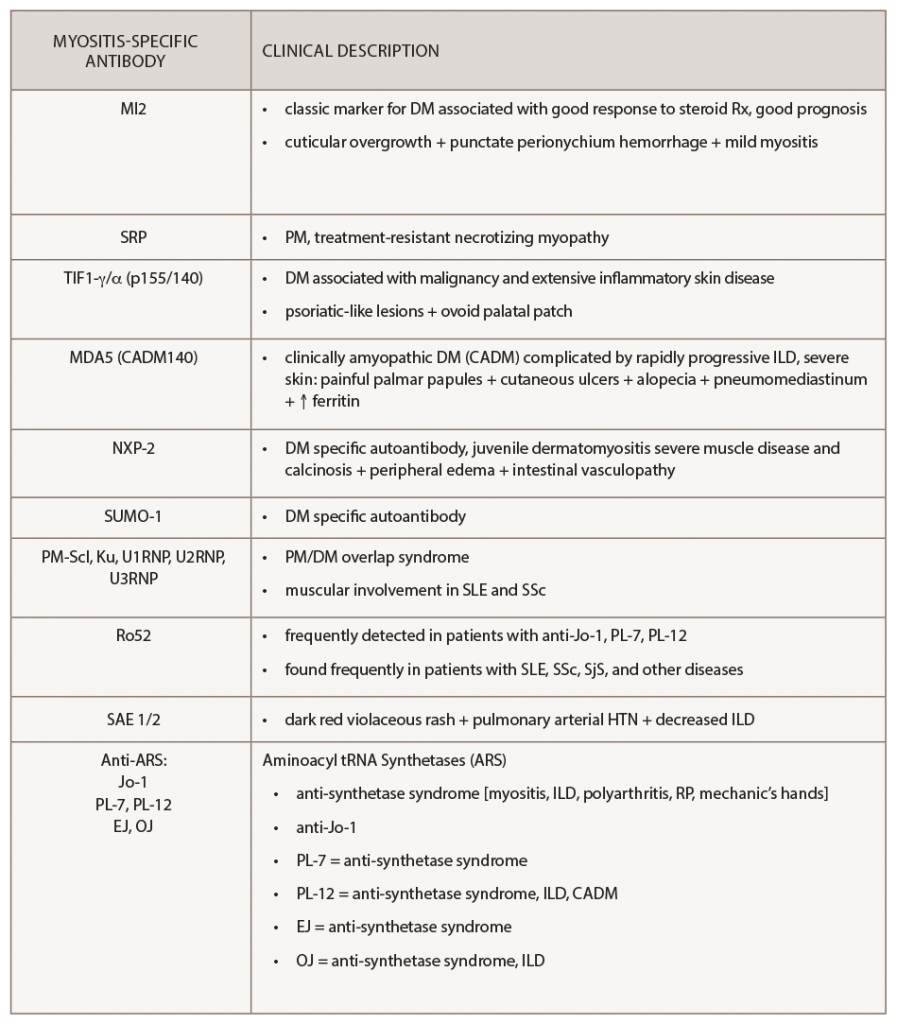
Interestingly, myositis-specific antibodies are closely associated with distinct clinical phenotypes and can help predict potential complications. Because myositis antibody panels can take up to several weeks to return, recognition of these clinical manifestations can identify disease subtypes early and help clinicians screen for associated complications. Recent studies have analyzed serologic data from inflammatory myositis cohorts, which have helped establish some of the clinical phenotypes associated with specific MSAs. An understanding of unique cutaneous manifestations associated with some of these antibody subtypes is emerging.
One of the leading researchers in this field is David Fiorentino, MD, PhD, professor of dermatology and director of a multidisciplinary rheumatology-dermatology clinic at Stanford University, Stanford, Calif. Dr. Fiorentino published some of the initial literature describing the clinical phenotype associated with anti-melanoma differentiation-associated gene 5 (anti-MDA5), a type of clinically amyopathic dermatomyositis.
Improved phenotyping of myositis patients in the past decade has enabled us to better understand unique clinical associations.
Retrospectively, plasma samples from 77 patients with dermatomyositis were collected from outpatient clinics at a tertiary medical center. Dr. Fiorentino’s group compared patients with anti-MDA5 antibodies (n=10) with those who were anti-MDA5 negative (n=67) and found the following clinical findings to be statistically associated with MDA5 positivity: interstitial lung disease, hand swelling, arthritis/arthralgias, clinically amyopathic dermatomyositis, skin ulceration, palmar papules, mechanic’s hands, panniculitis, alopecia, oral pain/ulcers and violaceous erythema of the elbow/knee.6 This systemic investigation ultimately helped identify the unique association of cutaneous ulcerations and palmar papules with MDA5 myositis.
More Nuanced Understanding
When initially pursuing this study, Dr. Fiorentino wanted to characterize the clinical features of patients with MDA5 myositis. He states none of the characteristics they thought may be present, such as Gottron’s papules or heliotrope rash, was seen in this subset of patients, and instead they noted an association with oral ulcers and palmar lesions.
Dr. Fiorentino explains how the findings started from the basis of simple clinical observation: “The most rewarding part is when we discovered this, it had nothing to do with mining a database and looking for a P value.”
He states that one of the areas of change in the past 10 years in myositis is a more nuanced understanding of the meaning of myositis antibodies. Each subgroup of antibodies has a lot of heterogeneity, Dr. Fiorentino says, “with interaction of genetics and environment ultimately leading to the particular phenotype.”
Lisa Christopher-Stine, MD, MPH, associate professor of medicine and neurology and director of the Johns Hopkins Myositis Center at Johns Hopkins University, Baltimore, says Dr. Fiorentino’s specialization at the intersection of dermatology and rheumatology offers a unique vantage point. “For years, I noticed a subset of patients with DM with rash on the palms, which I jokingly termed inverse Gottron’s papules,” she says, which later was recognized as the palmar papulosis seen in MDA5 disease. “It took the careful eye of a dermatologist [Dr. Fiorentino] to recognize those subtle cutaneous differences that define dermatomyositis beyond Gottron’s papules. David Fiorentino’s work was remarkable and pivotal, teaching us that not all dermatomyositis rashes are the same.”