Part 2 of a two-part series. This article will cover the role of pain processing in fibromyalgia (FM) and potential treatment methods. Part 1 appeared in the October 2009 issue (p. 1) and covered the history of the FM concept, current thinking on FM, and the epidemiology of the disease.
Augmented Pain and Sensory Processing Most Reproducible Feature
Once FM is established, the most consistently detected objective abnormalities involve pain and sensory processing systems. Since FM is defined in part by tenderness, considerable work has been performed exploring the potential reason for this phenomenon. The results of two decades of psychophysical pressure pain testing in FM have been very instructive.1
One of the earliest findings in this regard was that the tenderness in FM is not confined to tender points, but instead extends throughout the entire body.2,3 Theoretically, such diffuse tenderness could be either primarily due to a psychological factor (e.g., hypervigilance, where individuals are too attentive to their surroundings) or neurobiological influence factors (e.g., the plethora of factors that can lead to temporary or permanent amplification of sensory input).
Early studies typically used dolorimetry to assess pressure pain threshold, and concluded that tenderness was largely related to psychological factors, because these measures of pain threshold were correlated with levels of distress.3-5 To minimize the biases associated with “ascending” measures of pressure pain threshold (i.e., the individual knows that the pressure will be predictably increased), Petzke and colleagues performed a series of studies using more sophisticated paradigms using random delivery of pressures.6-8 These studies showed that: 1) the random measures of pressure pain threshold were not influenced by levels of distress of the individual, whereas tender point count and dolorimetry exams were; 2) patients with FM were much more sensitive to pressure even when these more sophisticated paradigms were used; 3) patients with FM were not any more “expectant” or “hypervigilant” than controls; and 4) pressure pain thresholds at any four points in the body are highly correlated with the average tenderness at all 18 tender points and four “control points” (the thumbnail and forehead). In addition to the heightened sensitivity to pressure noted in FM, other types of stimuli applied to the skin are also judged as more painful or noxious by these patients. Patients with FM also display a decreased threshold to heat,8-11 cold,10,12 and electrical stimuli.13
Gerster and colleagues were the first to demonstrate that patients with FM also display a low noxious threshold to auditory tones, suggesting a more global problem in sensory processing in some.14 A recent study by Geisser and colleagues used an identical random staircase paradigm to test FM patients’ threshold to the loudness of auditory tones and to pressure.15 This study found that patients with FM displayed low thresholds to both types of stimuli, and the correlation between the results of auditory and pressure pain threshold testing suggested that some of this was due to shared variance and some unique to one stimulus or the other. The notion that FM and related syndromes might represent biological amplification of all sensory stimuli has significant support from functional imaging studies that suggest that the insula is the most consistently hyperactive region, as discussed below. This region plays a critical role in sensory integration, with the posterior insula serving a purer sensory role, and the anterior insula associated with the emotional processing of sensations.16-18
These same findings of hyperalgesia and allodynia have been noted in most of the other conditions acknowledged to be part of this continuum, including irritable bowel syndrome (IBS), temporomandibular joint disorder, tension type headache, idiopathic low back pain, vulvodynia, and interstitial cystitis.19-25
Brain imaging studies also demonstrate the existence of central pain augmentation in FM, IBS, low back pain, and several other central sensitivity syndromes (CSSs).26-29
The Role of Specific Neurotransmitters
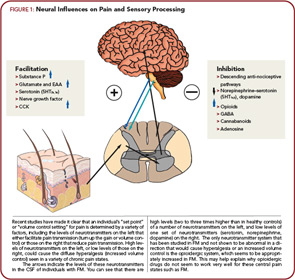
Rheumatologists might best understand pain and sensory processing by considering that this type of processing is controlled in a manner very similar to immune function. Just as high levels of proinflammatory cytokines, or low levels of antiinflammatory cytokines, can move an individual towards hyperimmune function, there are neurotransmitters that are similarly known to either increase or decrease pain transmission in the central nervous system (CNS). Overall, the analogy of an increased “volume control” or “gain” setting on pain and sensory processing is supported by studies from a variety of sources. Similar to essential hypertension, where a variety of root causes can lead to elevated systemic blood pressure, these disorders represent essential hypertension of pain and sensory processing pathways. Elevated levels of neurotransmitters that tend to be pro-nociceptive (see the left side of Figure 1, above) or reduced levels of neurotransmitters that inhibit pain transmission (see the right side of Figure 1) have a tendency to increase the volume control, and drugs that block neurotransmitters on the left or augment activity of those on the right will typically be effective treatments, at least for a subset of individuals with this spectrum of illness.
The arrows on Figure 1 indicate the direction of the abnormalities in these neurotransmitter levels, either in the cerebrospinal fluid (CSF) or brain, that have been identified to date in FM. In FM, there is evidence for increases in the CSF levels of substance P, glutamate, nerve growth factor, and brain derived neurotrophic factor, and low levels of the metabolites of serotonin, norepinephrine, and dopamine, any of which could lead to an increase in the volume control and augmented pain and sensory processing.30-33 The only neurotransmitter system that has been studied to date and not found to be out of line in a direction that would cause augmented pain transmission is the endogenous opioid system. Both CSF levels and brain activity by functional neuroimaging appear to be augmented, not reduced (as would cause augmented pain processing) in FM, which may be why opioidergic drugs do not work well to treat FM and related pain syndromes.34,35
Potential Role of Cytokines
Although the CSS conditions all were originally felt to be autoimmune or inflammatory diseases and then later felt not to be, recent findings are leading to a reconsideration of whether subtle inflammatory changes may be responsible for some of the symptoms seen. Immunological cascades have a role in the maintenance of central sensitivity and chronic pain which is enhanced through release of proinflammatory cytokines by CNS glial cells; thus, the traditional paradigm regarding inflammatory versus noninflammatory pain may gradually become less dichotomic. As may be expected in any complex biological system, a delicate apparatus of checks and balances is at work in the spinal transmission of pain. Multiple inhibitory transmitters act at the spinal level to reduce the volume of pain transmission. Serotonin, norepinephrine, enkephalins, dopamine, and gamma-aminobutyric acid (GABA) are among the better known players in this balance.36
Similar Treatments for Many CSS Entities
Several drug and nondrug therapies have been shown to be effective for most of the CSS disorders, further reinforcing that this may be a large overlapping disorder rather than several separate ones. Among classes of drugs, substantial data suggest that tricyclic compounds are effective for treating most of the conditions noted.37-39 Newer serotonin–norep – inephrine reuptake inhibitors such as duloxetine and tramadol have similarly been shown to be effective across a broad range of these conditions.40 Interestingly, duloxetine had much earlier been shown to be helpful in treating the pain associated with depression, which is not surprising. The alpha-2- delta ligands, such as pregabalin and gabapentin, are also being shown to be efficacious in a wide range of these entities.41
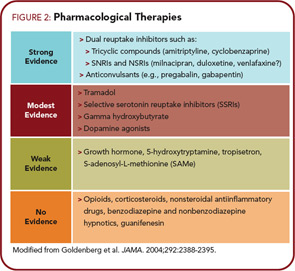
Figure 2 (above right) lists the classes of drugs and the level of evidence for their effectiveness in FM. In general, those drugs with the highest level of evidence of effectiveness in FM have also been shown to work in subsets of individuals with CSS. More importantly, drugs such as duloxetine are being shown to be effective in conditions such as osteoarthritis and low back pain, pointing out that the central mechanisms that are front and center in patients with syndromes such as FM may be also playing prominent roles in conditions previously thought to be peripheral pain syndromes. We have known for some time, however, that hyperalgesia and other central factors, as well as various other indicators of a wide range of “fibromyaglia-ness,” are present in conditions such as osteoarthritis and low back pain. It is of note that any one of these classes of drugs only works well in about a third of patients, which is entirely consistent that this is a strongly genetic—but polygenic— disorder and thus will need different treatments in different individuals. Going back to the “essential hypertension of pain processing pathway” analogy, just as we use eight to 10 classes of drugs acting in different body systems and at different molecular targets to control hypertension, and individuals may respond very well to one class of antihypertensive drug but not another, the same is true of CSSs. Individuals may only respond to one of these classes of drugs or may often be on several classes of centrally acting analgesics (e.g., a low dose of cyclobenzaprine at bedtime, pregabalin or gabapentin either just at bedtime or twice daily, and a serotonin– norepinephrine reuptake inhibitor such as duloxetine or milnacipran during the day). However, our current pharmacological armamentarium is not nearly as well developed for central pain as for essential hypertension, which is one likely reason that these syndromes are often difficult to treat.
Figure 2 also points out that classes of drugs such as NSAIDs and opioids that are quite effective for peripheral pain due to damage or inflammation in peripheral tissues are not effective analgesics in central pain states. There are even data suggesting that the use of opioids in individuals with central pain states may worsen their pain by leading to opioid-induced hyperalgesia that could augment and worsen the baseline hyperalgesia that may be playing a central pathogenic role in these conditions.
Just as many pharmacological therapies work across all or most of these conditions, similarly nonpharmacological therapies such as education, exercise, and cognitive behavioral therapy have been demonstrated to be effective across nearly all of the CSS conditions.42-44
Conclusion
In the past decades, our understanding of FM has evolved tremendously, and the study of FM has taught us about the mechanisms that may underlie chronic pain or other somatic syndromes in individuals without FM per se. A better understanding of the underlying mechanisms and most effective treatment for this spectrum of illness is critical to rheumatologists because, as Wolfe has taught us, many patients with rheumatic disorders have a little, or a lot, of “fibromyalgia-ness.” When this occurs, we need to treat both the peripheral and central elements of pain and other somatic symptoms.
Dr. Clauw is professor of anesthesiology and medicine (rheumatology) at the University of Michigan in Ann Arbor.
References
- Staud R, Spaeth M. Psychophysical and neurochemical abnormalities of pain processing in fibromyalgia. CNS Spectr. 2008;13(3 Suppl 5):12-17.
- Granges G, Littlejohn G. Pressure pain threshold in pain-free subjects, in patients with chronic regional pain syndromes, and in patients with fibromyalgia syndrome. Arthritis Rheum. 1993;36:642-646.
- Wolfe F, Ross K, Anderson J, Russell IJ. Aspects of fibromyalgia in the general population: Sex, pain threshold, and fibromyalgia symptoms. J Rheumatol. 1995;22:151-156.
- Wolfe F. The relation between tender points and fibromyalgia symptom variables: Evidence that fibromyalgia is not a discrete disorder in the clinic. Ann Rheum Dis. 1997;56:268-271.
- Gracely RH, Grant MA, Giesecke T. Evoked pain measures in fibromyalgia. Best Pract Res Clin Rheumatol. 2003;17:593-609.
- Petzke F, Gracely RH, Khine A, Clauw DJ. Pain sensitivity in patients with fibromyalgia (FM): Expectancy effects on pain measurements. Arthritis Rheum. 1999;42(9 (Supplement)):S342.
- Petzke F, Khine A, Williams D, Groner K, Clauw DJ, Gracely RH. Dolorimetry performed at 3 paired tender points highly predicts overall tenderness. J Rheumatol. 2001;28:2568-2569.
- Petzke F, Clauw DJ, Ambrose K, Khine A, Gracely RH. Increased pain sensitivity in fibromyalgia: Effects of stimulus type and mode of presentation. Pain. 2003;105:403-413.
- Gibson SJ, Littlejohn GO, Gorman MM, Helme RD, Granges G. Altered heat pain thresholds and cerebral event-related potentials following painful CO2 laser stimulation in subjects with fibromyalgia syndrome. Pain. 1994;58:185-193.
- Kosek E, Hansson P. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromialgia patients and healthy subjects. Pain. 1997;70:41-51.
- Geisser ME, Casey KL, Brucksch CB, Ribbens CM, Appleton BB, Crofford LJ. Perception of noxious and innocuous heat stimulation among healthy women and women with fibromyalgia: association with mood, somatic focus, and catastrophizing. Pain. 2003;102:243-250.
- Kosek E, Ekholm J, Hansson P. Sensory dysfunction in fibromialgia patients with implications for pathogenic mechanisms. Pain. 1996;68:375-383.
- Arroyo JF, Cohen ML. Abnormal responses to electrocutaneous stimulation in fibromyalgia. J Rheumatol. 1993;20:1925-1931.
- Gerster JC, Hadj-Djilani A. Hearing and vestibular abnormalities in primary fibrositis syndrome. J Rheumatol. 1984;11:678-680.
- Geisser ME, Gracely RH, Giesecke T, Petzke FW, Williams DA, Clauw DJ. The association between experimental and clinical pain measures among persons with fibromyalgia and chronic fatigue syndrome. Eur J Pain. 2007;11:202-207.
- Craig AD. Interoception: The sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500-505.
- Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55:377-391.
- Craig AD. Human feelings: Why are some more aware than others? Trends Co target=”_blank”gn Sci. 2004;8:239-241.
- Giesecke T, Gracely RH, Williams DA, Geisser ME, Petzke FW, Clauw DJ. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52:1577-1584.
- Rodrigues AC, Nicholas VG, Schmidt S, Mauderli AP. Hypersensitivity to cutaneous thermal nociceptive stimuli in irritable bowel syndrome. Pain. 2005;115:5-11.
- Maixner W, Fillingim R, Booker D, Sigurdsson A. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain. Pain. 1995;63:341-351.
- Langemark M, Jensen K, Jensen TS, Olesen J. Pressure pain thresholds and thermal nociceptive thresholds in chronic tension-type headache. Pain. 1989;38:203-210.
- Clauw DJ, Schmidt M, Radulovic D, Singer A, Katz P, Bresette J. The relationship between fibromyalgia and interstitial cystitis. J Psychiatr Res. 1997;31:125-131.
- Ness TJ, Powell-Boone T, Cannon R, Lloyd LK, Fillingim RB. Psychophysical evidence of hypersensitivity in subjects with interstitial cystitis. J Urol. 2005;173:1983-1987.
- Giesecke J, Reed BD, Haefner HK, Giesecke T, Clauw DJ, Gracely RH. Quantitative sensory testing in vulvodynia patients and increased peripheral pressure pain sensitivity. Obstet Gynecol. 2004;104:126-133.
- Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333-1343.
- Drossman DA. Brain imaging and its implications for studying centrally targeted treatments in irritable bowel syndrome: A primer for gastroenterologists. Gut. 2005;54:569-573.
- Rapps N, van Oudenhove L, Enck P, Aziz Q. Brain imaging of visceral functions in healthy volunteers and irritable bowel syndrome patients. J Psychosom Res. 2008;64:599-604.
- Giesecke T, Gracely RH, Grant MA, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613-623.
- Giovengo SL, Russell IJ, Larson AA. Increased concentrations of nerve growth factor in cerebrospinal fluid of patients with fibromyalgia. J Rheumatol. 1999;26:1564-1569.
- Sarchielli P, Mancini ML, Floridi A, et al. Increased levels of neurotrophins are not specific for chronic migraine: Evidence from primary fibromyalgia syndrome. J Pain. 2007;8:737-745.
- Russell IJ, Orr MD, Littman B, et al. Elevated cerebrospinal fluid levels of substance P in patients with the fibromyalgia syndrome. Arthritis Rheum. 1994;37:1593-1601.
- Russell IJ. Neurochemical pathogenesis of fibromyalgia. Z Rheumatol. 1998;57(Suppl 2):63-66.
- Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci. 2007;27:10000-10006.
- Baraniuk JN, Whalen G, Cunningham J, Clauw DJ. Cerebrospinal fluid levels of opioid peptides in fibromyalgia and chronic low back pain. BMC Musculoskelet Disord. 2004;5:48.
- Russell IJ. Neurotransmitters, cytokines, hormones and the immune system in chronic neuropathic pain. In: Wallace DJ, Clauw DJ, eds. Fibromyalgia and other central pain syndromes. Philadelphia: Lippincott Williams & Wilkins; 2005. p.63-79.
- Bryson HM, Wilde MI. Amitriptyline. A review of its pharmacological properties and therapeutic use in chronic pain states. Drugs Aging. 1996;8:459-476.
- van Ophoven A, Hertle L. Long-term results of amitriptyline treatment for interstitial cystitis. J Urol. 2005;174:1837-1840.
- Lynch ME. Antidepressants as analgesics: a review of randomized controlled trials. J Psychiatry Neurosci. 2001;26:30-36.
- Arnold LM. Duloxetine and other antidepressants in the treatment of patients with fibromyalgia. Pain Med. 2007;8(Suppl 2):S63-S74.
- Arnold LM, Goldenberg DL, Stanford SB, et al. Gabapentin in the treatment of fibromyalgia: A randomized, double-blind, placebo-controlled, multicenter trial. Arthritis Rheum. 2007;56:1336-1344.
- Bergman S. Management of musculoskeletal pain. Best Pract Res Clin Rheumatol. 2007;21:153-166.
- Deuster PA. Exercise in the prevention and treatment of chronic disorders. Womens Health Issues. 1996;6:320-331.
- Williams DA. Cognitive and behavioral approaches to chronic pain. In: Wallace DJ, Clauw DJ, eds. Fibromyalgia and other central pain syndromes. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. p. 343-352.