 SAN DIEGO—The proposed classification criteria for systemic lupus erythematosus (SLE), supported but not yet approved by the ACR and EULAR, were debuted on Nov. 7 at the 2017 ACR/ARHP Annual Meeting. An international steering committee developed and validated the criteria, with patient input and the consensus of more than 150 global SLE experts.
SAN DIEGO—The proposed classification criteria for systemic lupus erythematosus (SLE), supported but not yet approved by the ACR and EULAR, were debuted on Nov. 7 at the 2017 ACR/ARHP Annual Meeting. An international steering committee developed and validated the criteria, with patient input and the consensus of more than 150 global SLE experts.
Rheumatology researchers referred to both the 1997 ACR revised criteria and 2012 Systemic Lupus International Collaborating Clinics (SLICC) criteria when developing the new criteria, said Martin Aringer, MD, steering committee co-chair and chief of rheumatology at University Medical Center Carl Gustav Carus in Dresden, Germany.1 The committee set ambitious goals for the new criteria: to achieve higher sensitivity and specificity than previous criteria, account for lupus heterogeneity, incorporate manifestations to help identify early lupus patients and achieve worldwide consensus, using an expert-driven, data-driven methodology.2
Classification criteria are designed to identify a homogeneous set of patients for inclusion in clinical trials and translational studies, not to diagnose patients. “Diagnosis is individual. We look for the perfect therapy based on the individual prognosis. For classification, the aim is totally different. In diagnosis, we are entitled to use all the information we can gather on an individual patient. For classification, it has to be a feasible set of objective criteria,” Dr. Aringer said.
Although sensitivity is extremely critical for diagnosis to select therapies, it can be annoying in classification, because it is acceptable to exclude one or two patients from a large trial, Dr. Aringer said. “Diagnosis can be questioned again if the disease does not perform the way you expect, but in classification, you typically cannot go back and change the inclusion or exclusion. So specificity is very important.”
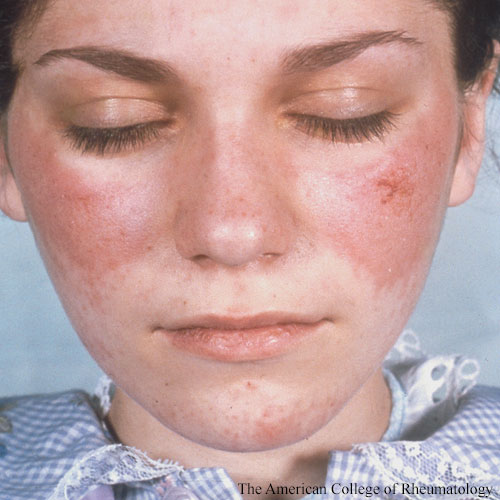
In classification, common features are most important, said Dr. Aringer. Although each lupus patient is unique, all have autoantibodies to nuclear antigens (ANA), as well as many other autoantibodies, and evidence of immune complex deposition from histology to complement to interferon signature. This leads to many different organ manifestations in SLE.
“ANA has very high sensitivity, but its specificity is actually pretty low. For anti-Smith antibodies, it’s the other way around. So it doesn’t make sense to have them side by side in the criteria,” said Dr. Aringer. They hypothesized that ANA would be the entry criterion and then tested if that was feasible.
Many conditions have overlapping features with SLE, “but if two of these attributes are found together, especially in a patient who also has autoantibodies, it’s usually SLE,” he said.

