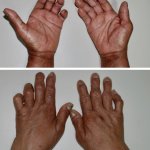
 Neuromuscular disorders, such as Charcot-Marie-Tooth type 1A, present many challenges to researchers, one of which is the lack of responsive outcome measures for clinical trials. Without an accurate and reliable means of monitoring disease progression, it becomes difficult for researchers to conduct successful experimental trials of new therapies. In the search for an objective measure, one clue has surfaced: Many of the neuromuscular disorders are characterized by chronic intramuscular fat accumulation that appears to contribute to the clinical presentation.
Neuromuscular disorders, such as Charcot-Marie-Tooth type 1A, present many challenges to researchers, one of which is the lack of responsive outcome measures for clinical trials. Without an accurate and reliable means of monitoring disease progression, it becomes difficult for researchers to conduct successful experimental trials of new therapies. In the search for an objective measure, one clue has surfaced: Many of the neuromuscular disorders are characterized by chronic intramuscular fat accumulation that appears to contribute to the clinical presentation.
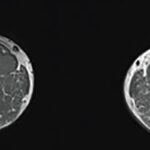
Although different diseases have different distributions and degrees of muscle abnormalities, the presence of the abnormalities and their correlation with symptoms are consistent across multiple neuromuscular disorders. Moreover, several studies have suggested that magnetic resonance imaging (MRI) may be a sensitive and non-invasive method of measuring skeletal muscle disease progression in various neuromuscular diseases. Specifically, investigators have used MRI to evaluate patients with Duchenne muscular dystrophy and have demonstrated that steroid use is associated with a reduction in transverse relaxation time constant (T2) in some patients or a variable effect on T2 in others.
In the case of inclusion body myositis associated with Charcot-Marie-Tooth type 1A, patients first present with minor symptoms. These symptoms progress over approximately 10 years to significant disability. Investigators asked whether it was possible to use MRI to track and measure these changes in muscle. Jasper M. Morrow, FRACP, at the UCL Institute of Neurology in London, and colleagues published the results of their prospective observational cohort study online on Nov. 5 in The Lancet Neurology.1 They evaluated muscle fat fraction using T2 and magnetic transfer ratio (MTR), and compared those measurements with relevant clinical functional tests. The investigators focused their efforts on the thigh and calf muscles of patients with Charcot-Marie-Tooth disease. Specifically, they analyzed data from single slices of thigh and calf blocks, using a small region of interest (ROI) for T2 and MTR sequences.
The researchers reported that MRI is a sensitive measure of disease progression in patients with Charcot-Marie-Tooth disease 1A and inclusion body myositis. Moreover, they found a strong clinical MRI correlation at both the overall participant level, as well as at the level of individual muscles. They also noted that, because the T2 of fat greatly exceeds the T2 of muscle water, T2 is affected by both fat fraction, as well as changes in tissue water distribution. Thus, MRI can be used to detect the muscle water changes that precede marked intramuscular fat accumulation.
The investigators concluded that the comprehensive MRC Centre MRI protocol appears to provide outcome measures that correlate closely with strength, function and disease severity. They went on to suggest that MRI biomarkers may be useful in experimental trials.
An accompanying editorial by Sean C. Forbes, PhD, research assistant professor at the University of Florida in Gainesville, Fla., and colleagues agreed about the potential value of MRI to monitor disease progression in clinical trials. They noted, however, that the method must first be standardized over several sites for MRI biomarkers to be successfully implemented in research.2
Lara C. Pullen, PhD, is a medical writer based in the Chicago area.
References
- Morrow JM, Sinclair CD, Fischmann A, et al. MRI biomarker assessment of neuromuscular disease progression: A prospective observational cohort study. Lancet Neurol. 2015 Nov 5. pii: S1474-4422(15)00242-2. doi: 10.1016/S1474-4422(15)00242-2. [Epub ahead of print]
- Forbes SC, Willcocks RJ, Rooney WD, et al. MRI quantifies neuromuscular disease progression. Lancet Neurol. 2015 Nov 5. pii: S1474-4422(15)00320-8. doi: 10.1016/S1474-4422(15)00320-8. [Epub ahead of print]