As soon as pediatric patients are diagnosed with juvenile idiopathic arthritis (JIA), they should also be screened for uveitis, says ophthalmologist Gary Holland, MD. Otherwise, the University of California, Los Angeles, provider says, “Kids who are diagnosed with JIA may not come to an ophthalmologist until they have vision-limiting complications.”
Uveitis is the most common extra-articular manifestation of JIA, and it is always chronic once it develops. Pediatric patients with uncontrolled chronic uveitis are at increased risk for cataracts and glaucoma, synechiae and vision loss. Until recently, recommendations for screening, monitoring and treating JIA patients were limited.
A First for the ACR/AF
On April 25, pediatric rheumatologist Sheila Angeles-Han, MD, MSc, and colleagues published the most comprehensive guideline for JIA-associated uveitis to date, containing 19 recommendations.1,2 The guideline appears in both Arthritis & Rheumatology and Arthritis Care & Research and is intended to aid clinical care for JIA patients at risk of, or already diagnosed with, uveitis.
“From a uveitis standpoint, this is the first guideline from the ACR and the Arthritis Foundation [AF],” says Dr. Angeles-Han, an associate professor at Cincinnati Children’s Hospital Medical Center and the University of Cincinnati. “There have been guidelines [published by others that] focused on treatment of pediatric uveitis, and there is a clinical report from the American Academy of Pediatrics that addresses ophthalmic screening in children with JIA, but there are not as many focused on ophthalmic monitoring, or on escalation and tapering of treatment in children with JIA-associated uveitis.”
The new guideline recommends treatment with methotrexate and the monoclonal tumor necrosis factor inhibitors (TNFi’s), adalimumab and infliximab, for management of uveitis in JIA patients when systemic treatment is warranted. The addition of biologic and non-biologic drugs reduces children’s risk for vision loss. Although the guideline calls for topical glucocorticoid use as an initial treatment to control uveitis-associated inflammation, it also recommends limiting pediatric exposure to glucocorticoids due to the potential for ocular toxicity.
“Often, the ophthalmologist is going to see the patient more often than the rheumatologist or pediatrician, so it’s important they are aware of what the medication options are and can counsel patients who need to know what to expect,” says Dr. Holland, who helped create the new guideline. “I think it’s important, if they’re going to be monitoring the patient, to have this body of information available.”
The Recommendation Process
The recommendations were generated through a comprehensive process that involved rheumatologists, ophthalmologists with experience with uveitis, patient and family representatives, and methodology experts.
The process included use of a methodology called Grading of Recommendations Assessment, Development and Evaluation (GRADE), which was used to rate the quality of available evidence as high, moderate, low or very low, relative to the patient/population, intervention, comparison and outcomes (PICO) questions the team derived.
“The main advantage [of GRADE] over the RAND methodology, used previously, is that it standardizes the process of moving from evidence to recommendations, leading to greater transparency around the process,” says Sarah Ringold, MD, MS, assistant professor of rheumatology at Seattle Children’s Hospital and a co-author of the guideline. Dr. Ringold also led the development of a new guideline for other aspects of JIA, including polyarthritis, sacroiliitis and enthesitis.3,4
Ophthalmic Monitoring
Among the strongest recommendations in the new JIA-associated uveitis guideline are those that involve ophthalmic monitoring of patients with JIA, particularly those at risk of developing or already diagnosed with uveitis. This, the authors say, is because the high risk of ocular complications that can lead to vision loss is high, but the risk of complications from ophthalmic exams is low.
These recommendations are included despite the fact that frequent monitoring can create strain for families.
“The treatment of the disease is often a bigger stressor for children than the disease itself,” says Dr. Holland, who with Dr. Angeles-Han and colleagues published a paper in the American Journal of Ophthalmology in 2018 describing the psychosocial elements for managing chronic anterior uveitis.5
However, says Dr. Angeles-Han, “In general, families agreed that scheduled ophthalmology visits for screening or monitoring of uveitis was important to achieve the most optimal visual outcomes.”
Guideline Objectives & Recommendations
Overall, the guideline team found a scarcity of good evidence in the literature, much of it rated low quality by the GRADE system, so the team developed its recommendations based on a combination of the best available data and consensus expert opinion. The guideline establishes terms, definitions and medication interventions, as well as critical outcomes for screening, monitoring and treatment. These include new diagnosis, loss of control of uveitis and side effects of systemic treatment.
Recommendations include:
- Conditional recommendation for ophthalmic screening every three months for children and adolescents with JIA at high risk for uveitis;
- Conditional recommendation to use topical glucocorticoids for short-term control rather than systemic glucocorticoids in children with JIA and active chronic anterior uveitis; and
- Strong recommendation for ophthalmic monitoring within one month of a change in glucocorticoids in children and adolescents with JIA and controlled uveitis who are tapering or discontinuing topical glucocorticoids.
(Editor’s note: See the table below for all the recommendations.)
Strong recommendations are those for which the guideline voting panel was confident the benefits of the action outweigh the risks and would apply to most or all patients.
Conditional recommendations are those for which the voting panel had less confidence in the risk–benefit assessment due to low quality evidence, with a preference for shared decision making because of known or expected variations in patient values and preferences. All treatment recommendations are conditional, the guideline authors say. For example, in children and adolescents with JIA and active chronic anterior uveitis starting systemic treatment, subcutaneous methotrexate is conditionally preferred over oral. So, too, is starting methotrexate and a monoclonal antibody TNFi immediately in cases of severe active chronic anterior uveitis and sight-threatening complications.
Treatment Tactics
The guideline also includes recommendations for tapering patients off treatment and for escalating treatment even when disease activity is low.
“Allowing disease to smolder at a low level is not good,” says Dr. Holland. “Even if a child is asymptomatic, ultimately, there’s a high risk of vision-limiting complications, like glaucoma or cataracts, so eye disease needs to be aggressively treated to suppress the activity.”
Dr. Angeles-Han adds, “In general, children with uveitis tend to have a flare in their disease when treatment is tapered too early,” so the team recommends that disease be well controlled for at least two years before initiating taper in JIA-associated uveitis patients, accompanied by frequent monitoring.
She hopes better studies of JIA-associated uveitis will ultimately offer other treatment recommendations and to gain a better sense of the risk factors associated with the development of uveitis and severe disease in JIA patients.
Teamwork & Collaboration
Dr. Angeles-Han, Dr. Ringold and Dr. Holland each believe in the importance of teamwork and collaboration in the management of JIA patients with uveitis. “Patients deserve to be managed in a team approach,” says Dr. Holland. “Rheumatologists are the experts in the drugs and their side effects, but ophthalmologists are the experts in monitoring the effects of drugs on uveitis, so each group should understand what the other is doing.”
“I think what’s important—and what we are always going to go back to—is the ophthalmologic exam,” Dr. Angeles-Han says. “That’s where we need to have collaboration and communication between both subspecialists. … We have to all be on same page with screening and monitoring schedules and treatment.”
Kelly April Tyrrell writes about health, science and health policy. She lives in Madison, Wis.
References
- Angeles-Han ST, Ringold SR, Beukelman T, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the screening, monitoring, and treatment of juvenile idiopathic arthritis-associated uveitis. Arthritis Care Res(Hoboken). 2019 Apr 25. doi: 10.1002/acr.23871. [Epub ahead of print]
- Angeles-Han ST, Ringold SR, Beukelman T, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the screening, monitoring, and treatment of juvenile idiopathic arthritis-associated uveitis. Arthritis Rheumatol. 2019 Apr 25. doi: 10.1002/art.40885. [Epub ahead of print]
- Ringold SR, Angeles-Han ST, Beukelman T, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the treatment of juvenile idiopathic arthritis: Therapeutic approaches for non-systemic polyarthritis, sacroiliitis, and enthesitis. Arthritis Care Res. 2019 Apr 25. doi: 10.1002/acr.23870. [Epub ahead of print]
- Ringold SR, Angeles-Han ST, Beukelman T, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the treatment of juvenile idiopathic arthritis: Therapeutic approaches for non-systemic polyarthritis, sacroiliitis, and enthesitis. Arthritis Rheumatol. 2019 Apr 25. doi: 10.1002/art.40884. [Epub ahead of print]
- Parker DM, Angeles-Han ST, Stanton AL, Holland GN. Chronic anterior uveitis in children: Psychosocial challenges for patients and their families. Am J Ophthalmol. 2018 Jul;191:xvi–xxiv. doi: 10.1016/j.ajo.2018.03.028.
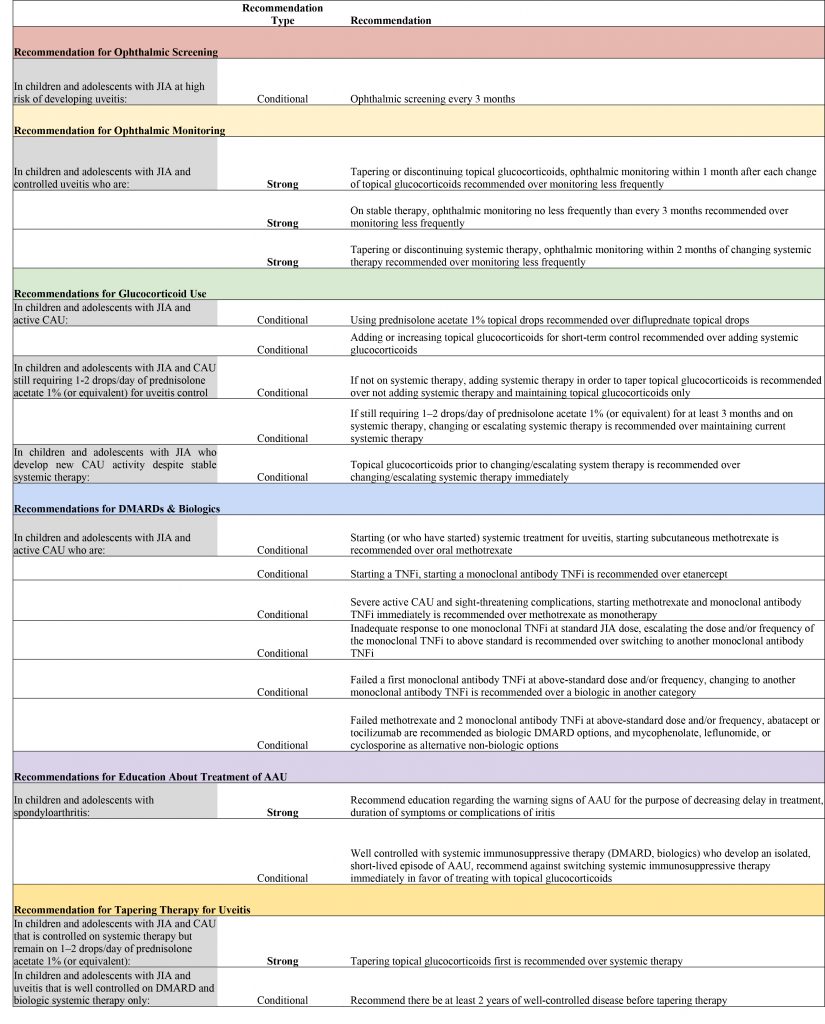
Table notes: Each recommendation had very low-quality level of evidence.
High-risk children are those with oligoarthritis, polyarthritis (rheumatoid factor negative), psoriatic arthritis, or undifferentiated arthritis who are also anti-nuclear antibody positive, younger than 7 years of age at JIA onset, and have JIA duration of four years or less.
Definition of new CAU activity: no prior uveitis or loss of control of previously controlled uveitis.
Strong recommendations are those for which the guideline voting panel was confident the benefits of the action outweigh the risks and would apply to most or all patients. Conditional recommendations are those for which the voting panel had less confidence in the risk-benefit assessment, with a preference for shared decision making.
Table key: JIA = juvenile idiopathic arthritis; PICO = patient/population, intervention, comparison and outcomes; CAU = chronic anterior uveitis; DMARDs = disease- modifying anti-rheumatic drugs; TNFi = tumor necrosis factor inhibitor; AAU = acute anterior uveitis.