Recent epidemiologic studies suggest a growing CPPD prevalence in developed countries, not simply due to increased longevity of the population, but to iatrogenic factors as well. Examples include hypomagnesemia, promoted by increased prescription of loop and thiazide diuretics, proton pump inhibitors, and calcineurin inhibitors (cyclosporine, tacrolimus), and by iatrogenic short bowel syndrome.6
Advances in CPPD Pathogenesis and Molecular Genetics
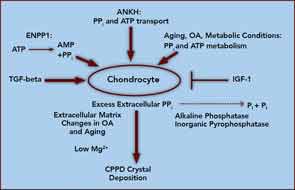
At the core of crystal deposition in idiopathic and other forms of CPPD is dysregulated PPi metabolism, accompanied by altered chondrocyte differentiation and function (see Figure 1).7,8 Articular cartilage, unlike growth-plate cartilage, is specialized to avoid the process of matrix calcification. Relatively copious output of PPi by chondrocytes physiologically functions to limit basic calcium phosphate crystal growth. This protective mechanism becomes deleterious by excessive PPi output in CPPD.7,8 Factors responsible for upregulating PPi generation in the aging joint and in OA are likely heterogeneous, but most function through increasing articular ectonucleotide pyrophosphatase phosphodiesterase (ENPP1) and the multiple-pass, transmembrane protein ankylosis protein homolog human gene (ANKH).7,8
ENPP1 generates intracellular and extracellular PPi via hydrolysis of ATP and other nucleoside triphosphates, and articular ecto–nucleotide pyrophosphatase/phosphodiesterase (ecto-NPP) activity is substantially elevated in both CPPD and OA.8 Intracellular PPi and ATP are transported to the extracellular space by processes involving ANKH, whose expression rises in OA chondrocytes.7,8
Players in aging- and OA-associated alterations in PPi metabolism also include dysregulated chondrocyte responses to growth factors (See Figure 1).8-10 Transforming growth factor-β (TGF-β) increases, insulin-like growth factor-1 (IGF-1) decreases chondrocyte extracellular PPi, and calcium (Ca2+) potentiates the effects of TGF-β on PPi.8-10 Certain chondrocyte responses to TGF-β—including PPi generation—increase in aging cartilage, possibly via known changes in TGF-β receptor subunit expression. Moreover, IGF-1 resistance increases in aging and OA cartilage, due to a variety of factors.8-10
Ambient cartilage magnesium (Mg2+) concentration, and multiple chondrocyte extracellular matrix components, including collagens II and I, proteoglycans, and osteopontin, also modulate crystal deposition in CPPD, and Mg2+ helps determine whether more inflammatory monoclinic or less inflammatory triclinic CPPD crystals are formed.8,11 We now recognize that the inflammatory responses to crystals in CPPD, as in gout, are mediated by NOD-like receptor, pyrin domain containing 3 (NLRP3) inflammasome activation and consequent caspase-1 activation, and interleukin 1-β (IL-1β) maturation and secretion.12